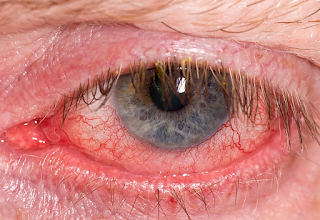
Dry Eye Disease is Similar to Severe Angina.
Severe Dry Eye Disease is
Worse than Disabling Hip Fracture
It is time for insurance companies to take dry eye disease seriously! It is ridiculous they do not cover dry eye treatment procedures which have been shown to provide relief and prevent worsening pain.
Dry Eye Syndrome and Dry Eye Disease are causing terrible pain, anxiety from the pain and depression from the pain. More research is needed in these fields, and patients need to demand help from their insurance companies for dry eye treatments, such as Lipiflow, Intense Pulse Light, and Meibomian Gland Probing. We know these options work for many patients but many patients do not have the means to pay for them.
I know many surgeons who do not even mention these options to patients because insurance does not pay for it. The problem is that if they do not have these procedures and the patient looses the majority of meibomian glands, there is little we can do to help. Treatments such as lacrimal gland stimulation (#20 on my Step Ladder) is costing about $15000 right now.
It is a race against time to save the Meibomian Glands in these patients and insurances need to help their patients as well.
Sandra Lora Cremers, MD, FCS
I must include this link for all my patients who have asked me about Dry Eye Glasses. I first saw these a few months ago in a computer programmer who could not stand the perfumes around him in his open office: these fragrances really irritated his eyes. He said this was the only thing that helped him. It also helped with the allergy season which made his eyes worse.
Panoptx is the brand. When it is really bad, he will put more tape around the edges, his eyes get that irritated even with a humidifier near him at all times. His meibomian gland score is 2 out of 3 with few glands left.
Abstract
Dry eye disease (DED) is a growing public health concern causing ocular discomfort, fatigue and visual disturbance that interferes with quality of life (QoL), including aspects of physical, social, psychological functioning, daily activities and workplace productivity. This paper assesses the current understanding of the impact of DED on QoL and vision. The full impact of DED on a patient’s QoL is not easily quantifiable, but several methods and techniques have been evaluated to measure the decreased quality of vision from DED, and a number of questionnaires have been developed to quantify the impact of DED on various aspects of patient QoL. We summarize available evidence on the impact of DED based on a review of published literature.
Keywords:
Published in final edited form as:
PMCID: PMC3660735
NIHMSID: NIHMS456226
Dry Eye Disease: Impact on Quality of Life and Vision
See other articles in PMC that
cite the published article.
Use of utility assessments in DED
Utility assessment is a formal method for quantifying and understanding the relative impact of a given health state or disease on patients. One of the advantages of this technique is that utility scores that are anchored at perfect health (utility=1) and death (utility=0) can be compared across various health outcomes using a time trade-off (TTO) method
39. Two studies used the technique of utility assessment to estimate the QoL impact of DED, finding that for patients with severe DED, the impact of their disease on their lives was similar to the impact of moderate to
severe angina40, 41. Schiffman found that for the most severe DED cases (requiring tarsorrhaphy), the impact was worse than that reported for disabling hip fracture
41. Buchholz P et al. from the United Kingdom showed a similar result; forty-four patients with moderate to severe dry eye were surveyed via interactive utility assessment software. Utility values were measured by TTO and Standard Gamble (SG) methods. Using TTO, the mean score for asymptomatic dry eye (0.68) was similar to that for “some physical and role limitations with occasional pain” and severe DED requiring surgery scored 0.56, similar to hospital dialysis (0.56-0.59) and severe angina (0.5). Utilities described for scenarios of dry eye severity levels were slightly higher (less severe impact) for patients self-reported as mild-to-moderate versus those self-reported as severe
40
Allergan (NYSE:
AGN) said today that it’s on track for an FDA submission this year for the Oculeve nasal neurostimulation device for dry eye it bought last year.
Today the company said a pair of pivotal trials passed their safety and efficacy endpoints, clearing the way for a pre-market approval submission in the 2nd half of the year.
The 48-patient
OCUN-009 trial evaluated intra- and extra-nasal use of the Oculeve device compared with sham intra- and extra-nasal treatments at 6 months, using a safety endpoint of device-related adverse events and an efficacy endpoint of tear production during treatment with tear production during the sham applications. Allergan said the study met the efficacy endpoint.
The 2nd study,
OCUN-010, tracked 97 subjects who used the Oculeve device for 6 months, with a safety endpoint of device-related adverse events and the same efficacy endpoint as OCUN-009. Allergan said all device-related adverse events were mild and no patients stopped treatment due to an adverse event, and that the efficacy endpoint was also met.
“We are excited with the outcome of these 2 sets of pivotal data,” chief R&D officer David Nicholson said in prepared remarks. “The Oculeve intranasal tear neurostimulator is a novel approach and has the potential to help patients suffering from dry eye by increasing their natural tears. This device is part of Allergan’s strong eye care development pipeline and will complement our leading dry eye treatment Restasis. This is a major step forward in providing a promising new option for eye care professionals and their patients with dry eye disease.”.