Has anyone tried Breast Milk for Dry Eyes in humans in a study?
Not that I can find, but it has been done in rats (see reference 1 **)
There are many reports of moms using breast milk for their own eye issues like conjunctivitis and their kids. I also have a friend that used it for herself and for her child for “conjunctivitis” or “pink eye” and it helped. But these are case reports. I could not find a randomized, controlled, prospective study on this and I doubt anyone will ever do this as pharmaceutical companies would not want to pay for such a study.
The risk of using your own breast milk for any eye condition is very low. The risk of using the breast milk of a donor is also low but not zero given a low but not zero risk of infection. HIV can be transmitted through breast milk, for instance, but there
The author notes:
“Topical human milk treatment demonstrated the ability
to preserve corneal epithelial thickness in the BAK-induced
dry eye mouse model and holds the potential to be an effective
treatment for DES in humans.
In addition, human milk may
provide a viable alternative for DES treatment for patients
more inclined to TEM (Traditional eye medicine) or those with limited health care
access.
Further studies are indicated to determine whether
human milk can be safely used to treat dry eye in humans,
whether the benefit conferred in this study is applicable to
more easily procured animal milk, and whether processing
and long-term storage, such as in pasteurization, affect efficacy. Moreover, studies should be directed at elucidating the
active components of human milk and their mechanisms of
action, potentially expanding future treatment options for
DES.”
I could not find a randomized, controlled, prospective study on this and I doubt anyone will ever do this as pharmaceutical companies would not want to pay for such a study.
The risk of using your own breast milk for any eye condition is very low. The risk of using the breast milk of a donor is also low but not zero.
Board-certified pediatrician Jarret Patton, MD, FAAP, is noted as saying that there’s mixed evidence on whether breast milk can actually treat pink eye or ear infections, despite the positive results these moms are seeing. “There have been studies that point to the potential of antibiotic properties of breast milk, but this certainly would not be in my treatment arsenal for ear and eye infections,” he says. “In fact, for the majority of inner ear infections, the milk wouldn’t get through the tympanic membrane to get to the infection. However, it is unlikely to be harmful.” https://thestir.cafemom.com/parenting_news/210303/breast-milk-treating-pink-eye/214491/others_think_its_dangerous_to/9
Of noted: a low-tech method, flash-heat treatment appears to preserve the bacteriostatic activity of human milk.158 Much work remains to be done regarding the bioactivity of human milk components following milk treatment.162
References:
1. **
Effect of human milk as a treatment for dry eye syndrome in a mouse model
Jose L. Diego, Luke Bidikov, […], and Emily A. McCourt
Abstract
Purpose
Dry eye syndrome (DES) affects millions of people worldwide. Homeopathic remedies to treat a wide variety of ocular diseases have previously been documented in the literature, but little systematic work has been performed to validate the remedies’ efficacy using accepted laboratory models of disease. The purpose of this study was to evaluate the efficacy of human milk and nopal cactus (prickly pear), two widely used homeopathic remedies, as agents to reduce pathological markers of DES.
Methods
The previously described benzalkonium chloride (BAK) dry eye mouse model was used to study the efficacy of human milk and nopal cactus (prickly pear). BAK (0.2%) was applied to the mouse ocular surface twice daily to induce dry eye pathology. Fluorescein staining was used to verify that the animals had characteristic signs of DES. After induction of DES, the animals were treated with human milk (whole and fat-reduced), nopal, nopal extract derivatives, or cyclosporine four times daily for 7 days. Punctate staining and preservation of corneal epithelial thickness, measured histologically at the end of treatment, were used as indices of therapeutic efficacy.
Results
Treatment with BAK reduced the mean corneal epithelial thickness from 36.77±0.64 μm in the control mice to 21.29±3.2 μm. Reduction in corneal epithelial thickness was largely prevented by administration of whole milk (33.2±2.5 μm) or fat-reduced milk (36.1±1.58 μm), outcomes that were similar to treatment with cyclosporine (38.52±2.47 μm), a standard in current dry eye therapy. In contrast, crude or filtered nopal extracts were ineffective at preventing BAK-induced loss of corneal epithelial thickness (24.76±1.78 μm and 27.99±2.75 μm, respectively), as were solvents used in the extraction of nopal materials (26.53±1.46 μm for ethyl acetate, 21.59±5.87 μm for methanol). Epithelial damage, as reflected in the punctate scores, decreased over 4 days of treatment with whole and fat-reduced milk but continued to increase in eyes treated with nopal-derived materials.
Conclusions
Whole and fat-reduced human milk showed promising effects in the prevention of BAK-induced loss of corneal epithelial thickness and epithelial damage in this mouse model. Further studies are required to determine whether human milk may be safely used to treat dry eye in patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3586783/#!po=8.33333
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5017541/pdf/mv-v22-1095.pdf
- Review
- Open Access
- Published:
Recent advances of exosomes in immune-mediated eye diseases
Stem Cell Research & Therapy 10, Article number: 278 (2019)
Abstract
Exosomes, nanosized extracellular vesicles of 30–150 nm, are shed by almost all cell types. Bearing proteins, lipids, RNAs, and DNAs, exosomes have emerged as vital biological mediators in cell-to-cell communication, affecting a plethora of physiological and pathological processes. Particularly, mounting evidence indicates that immunologically active exosomes can regulate both innate and adaptive immune responses. Herein, we review recent advances in the research of exosomes in several immune-mediated eye diseases, including Sjögren’s syndrome (SS) dry eye, corneal allograft rejection, autoimmune uveitis, and age-related macular degeneration (AMD). Additionally, we discuss the potential of exosomes as novel biomarkers and drug delivery vesicles for the diagnosis and treatment of eye diseases.
Introduction
Exosomes were first described as 50-nm diameter-sized vesicles secreted from maturing sheep reticulocytes in the early 1980s [1, 2]. These nanovesicles sparked scientists’ interest, as they appeared to function from cellular garbage disposals to potent intercellular communication mediators. Typically, exosomes are a subtype of extracellular vesicles (EVs) (30–150 nm) secreted by almost all cell types [3, 4]. They widely exist in numerous biological fluids including serum, urine, breast milk, tear fluid, vitreous humor, saliva, and aqueous humor, under both healthy and pathological conditions [5, 6]. Encapsulated in a bilayer membrane, exosomes are enriched in various bioactive molecules, including proteins, lipids, RNAs (mRNA, circular RNA, microRNA, long noncoding RNA), and DNAs (genomic DNA, cDNA, and mitochondrial DNA) [7,8,9]. These molecular components are capable of inducing functional responses in recipient cells and are extraordinarily variable depending on the cellular origin and cell exposure context [10,11,12,13]. By transferring these functional molecules between cells, exosomes act as potent mediators in intercellular communication and participate in numerous physiological and pathological processes [14]. Exosomes from both immune cells and non-immune cells exert pivotal roles in the regulation of immunity [15] and have been reported to be involved in the development and treatment of inflammatory and autoimmune diseases [16, 17].
The eye, a unique sensory organ of vision, is regarded as an immune-privileged site that prevents immunogenic inflammation [18]. Still, there are several inflammatory and immune-mediated diseases which involve the anterior or posterior segment of the eye, even in severe cases resulting in sight-threatening conditions, such as Sjögren’s syndrome (SS) dry eye, corneal allograft rejection, uveitis, and age-related macular degeneration (AMD) [19,20,21]. Of these diseases, the action of immune cells and the expression of pro-inflammatory cytokines and chemokines induce local inflammatory responses which ultimately cause ocular tissue damage. Although therapeutic strategies have undergone substantial transformation, there are still some challenges remaining [22, 23].
In this review, we highlight and discuss the recent research advances about exosomes in several immune-related eye diseases and their potential as biomarkers and drug delivery vesicles in the eye.
Biogenesis and function of exosomes
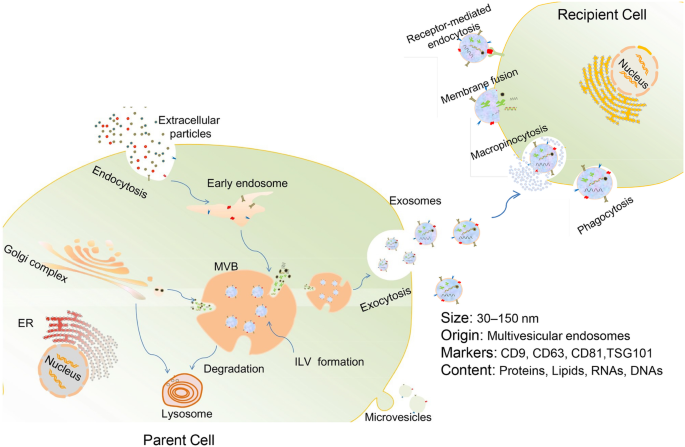
Exosome generation starts with the invagination of the plasma membrane to form early endosomes. As the early endosomes mature, intraluminal vesicles (ILV) are produced in the lumen of the late endosomes (also called multivesicular bodies, or MVBs). MVBs eventually fuse with the plasma membrane and release their internal contents as exosomes. Alternatively, some MVBs are destined for degradation inside of lysosomes [3, 14] (Fig. 1). Cargoes assembled into exosomes are sorted through several molecular machinery, including the endosomal sorting complex required for transport (ESCRT) machinery (containing ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) and ESCRT-independent machinery (involving lipids, syndecan, and syntenin) [24, 25]. In addition, the Rab family of small GTPase proteins (such as Rab27a and Rab27b), SNARE (soluble N-ethylmaleimide-sensitive fusion attachment protein receptor) complexes, and cytoskeleton act as important modulators of exosomes secretion [24]. However, in spite of the heightened interest in this field, the mechanisms that control exosome biogenesis and secretion are still not exhaustive.
Biogenesis, release, and internalization of exosomes. Exosomes originate from early endosomes which then mature to late endosomes or MVBs. Numerous proteins, nucleic acid, and lipids are selectively encompassed in exosomes during the formation of ILV inside MVBs via the invagination of the endosomal membrane. Eventually, exosomes are released outside the cell upon fusion of MVBs with the plasma membrane. The internalization of exosomes by recipient cells can be mediated by receptor-mediated endocytosis, macropinocytosis, phagocytosis, or direct fusion of exosomes with cell membrane
Once released, exosomes can interact with specific recipient cells. It appears that exosome targeting specificity is based on the particular combination of exosomes and acceptor cells [24]. Studies have identified that the expression of phosphatidylserine receptors, integrins, tetraspanins, lectins, glycans, and other adhesion molecules on exosome surface contributes to this process [26, 27]. Exosomes can transmit information to target cells via internalization through macropinocytosis, phagocytosis, receptor-mediated endocytosis, or membrane fusion [28,29,30], or via acting on their cell surface, without delivery of their cargos [31] (Fig. 1). Nevertheless, the more specific cellular and molecular basis for exosome targeting is still undetermined.
The function of exosomes was unknown until 1996, when it was found that exosomes derived from Epstein-Barr virus (EBV)-transformed B cell lines induced major histocompatibility complex (MHC) class II-restricted T cell responses in an antigen-specific manner, hinting the possible role of exosomes as mediators of immune responses [32]. Since then, intensive research has been devoted to delineating their roles in immunomodulation. It is now clearly understood that immunologically active exosomes can regulate both innate and adaptive immunity [33, 34]. Exosomes generated by immune cells have been studied extensively. For instance, exosomes from antigen-presenting cells including dendritic cells (DCs), B cells, and macrophages carry surface MHCI and MHCII molecules and thus directly stimulate CD8+ and CD4+ T cell responses, respectively [15, 35]. Besides, Okoye et al. demonstrated that Let-7d-containing exosomes derived from primary regulatory T cells (Tregs) inhibited Th1 cell responses by targeting Cox-2 in a mouse model of colitis [36]. Of note, exosome secretion in immune cells is regulated by cell context. For example, exosome release in DCs and B cells is increased after cognate T cell interactions [37,38,39], and mast cells produce more EVs in response to cross-linking of the high-affinity Fc receptor for IgE or exposure to calcium ionophores [40]. Aside from immune cell-derived exosomes, exosomes secreted by nonimmune cells such as tumor and mesenchymal stem cells (MSCs) have gained great attention in recent years. Tumor-cell-derived exosomes can travel to the draining lymph node, where they inhibit T cell activation by presenting programmed death-ligand 1 (PD-L1) and thus promote tumor progression [41]. Mesenchymal stem cells-derived exosomes (MSC-Exos) have been shown to enhance the differentiation of immunosuppressive cells such as M2 macrophages and Tregs, or inhibit proliferation of natural killer cells or T lymphocytes [42]. For instance, Zhao et al. discovered that mouse bone marrow-derived MSC-Exos modulated macrophage polarization by transferring miR-182, which targeted TLR4/NF-κB/PI3K/Akt signaling [43]. More attractively, several studies proposed that inflammatory stimulation increased secretion of MSC-Exos and even enhanced their anti-inflammatory and immunosuppressive properties [44,45,46]. However, although exosomes possess versatile biological functions including immunomodulation [47], pro-regeneration [48], anti-inflammation [49], and tumor growth regulation [50] (Table 1), the field of exosome research in eye diseases currently remains relatively less explored.
Exosomes in immune-mediated eye diseases
Sjögren’s syndrome (SS) dry eye
Sjögren’s syndrome (SS), a multisystem autoimmune disease, is characterized by lymphocytic infiltration in salivary and lacrimal glands (LGs) and the presence of various autoantibodies (such as anti-Ro(SS-A) or anti-La(SS-B)), resulting in oral and ocular dryness [67, 68]. This condition leads to one of the most severe subtypes of dry eye diseases [20]. Activation of both innate and adaptive immune pathways, such as interferon (IFN) signatures, B cell activating factor (BAFF)/BAFF receptor axis, and NF-kB signaling, contributes to the pathogenesis of SS [69, 70].
Salivary gland epithelial cells (SGECs) in SS play active roles in the autoimmune and inflammatory responses by virtue of the constitutive or inducible expression of diverse immunoactive factors, such as BAFF, several Toll-like receptors (TLRs), and autoantigenic ribonucleoproteins (RNPs) [71, 72]. Lymphocytic infiltrates consisting primarily of CD4+ T cells and B cells occur proximally to and frequently invade epithelial cells [73, 74], suggesting the interaction between epithelial and immune cells. One previous study demonstrated that the autoantigenic Ro/SS-A, La/SS-B, and Sm RNPs were present in exosomes which were released continuously by SGECs, indicating that intracellular autoantigens were transferred to autoreactive lymphocytes via RNP-containing exosomes. However, this release is not restricted to SS-derived cells [51]. Besides, as EBV typically infects B cells, one study proposed that EBV-miRBART13-3p could be transferred via exosomes from B cells to SGECs. This functional miRNA targeted aquaporin 5 (AQP5) and stromal interacting molecule 1 (STIM1), which could significantly impact salivary secretion. However, the authors did not mention the effect on the function of LGs [52].
The LGs are primarily responsible for the aqueous layer of the tear film. LG dysfunction is mainly due to the infiltration of immune cells [75]. Our research team has verified that MSC administration efficiently alleviated induced autoimmune dacryoadenitis in rabbit models, which closely mimic human SS [76]. It is noted that MSC-Exos mediate the immunosuppressive effects of their parent cells and are deemed as promising surrogates for MSC-based therapy [33]. Ongoing studies in our laboratory recently demonstrated that subconjunctivally administered MSC-Exos efficiently improved clinical evaluations and diminished the inflammation in lacrimal glands of diseased rabbits, compared with those treated with saline. The therapeutic effects may partially be ascribed to their modulatory effects on lacrimal macrophage polarization and enhancement of Treg and Th2 responses via targeting NF-kB signaling. Therefore, MSC-Exos presumably provide a very promising cell-free therapy for SS dry eye. In addition, the role of exosomes in interactions between lymphocytes and LG epithelial cells remains unexplored, calling for extensive research.
Corneal allograft rejection
Corneal transplantation is the most prevalently performed type of tissue grafting globally. To enhance corneal graft survival, considerable efforts have been devoted to building effective strategies [77]. Although cornea as an avascular transparent tissue enjoys the relative privilege of immunity, the major cause of corneal graft failure reported is allogeneic rejection, which is ascribed to the adaptive immune response initiated through recognition of donor MHC antigens by recipient T cells after transplantation [78, 79]. EVs, including exosomes, released by donor cells are partly responsible for this type of allorecognition [80]. Howbeit, they also contribute to allograft tolerance under certain circumstances. It has been reported that EVs from a specific population of CD4+CD25− Tregs generated in vitro could prolong kidney allograft survival, which was mediated by their unique cargo, specific miRNAs, and inducible nitric oxide synthase (iNOS) enzyme [53]. Moreover, MSC-Exos loaded with specific small RNAs successfully improved islet transplantation [54]. These encouraging results suggest that exosomes from specific immunosuppressive cell populations serve as a potentially effective tool to promote immune tolerance in graft survivals such as corneal graft.
For decades, severe global shortfall of donated human corneas has been an ongoing challenge that should not be ignored [81]. To address this, new biomaterials, such as collagen gels, synthetic polymers, and tissue-engineered scaffolds, have been developed to repair, regenerate, or replace the damaged cornea [82]. Jangamreddy et al. found that one kind of peptide analogs as alternatives to collagen promoted regeneration of corneal tissue by stimulating in-growing corneal epithelium cells to secrete EVs for generating matrix components [55]. During corneal wound healing, mouse corneal epithelial cell-derived exosomes induced fibroblast proliferation and transformation of keratocytes to myofibroblasts, mediating intercellular communication between the corneal epithelium and stroma [56]. Besides, exosomes derived from normal human corneal limbal keratocytes were found to greatly enhance proliferation and wound healing rates of primary limbal epithelial cells, likely via activating Akt signaling [57]. One recent study revealed that human corneal MSC-Exos were capable of accelerating corneal epithelial wound healing [58]. Together, the available results indicate that exosomes are vital biological mediators of regeneration [83] and provide new insights into the therapeutic strategies for corneal injury and transplant rejection.
Autoimmune uveitis
Autoimmune uveitis, an inflammation of the uvea (iris, ciliary body, and choroid tissue) and even adjacent tissues (vitreous humor, optic nerve and retina), can occur either alone or secondary to systemic syndrome [84]. The autoimmune causes are mainly due to inappropriate immune responses mediated by pathogenic T cells [85]. Pathogenic Th17 cells and their related inflammatory cytokines coordinately act as potent inducers of tissue inflammation [86, 87]. Innate immune cells such as DCs, monocytes/macrophages, γδT cells, natural killer (NK) cells, and NKT cells also actively participate in shaping the effector T cell responses in autoimmune uveitis [88, 89].
During the inflammatory processes, particularly in posterior uveitis, retinal pigment epithelium (RPE) cells may get damaged [90]. RPE cells have been revealed to have immunosuppressive properties, including induction of Tregs and inhibition of Th17 and Th22 cell differentiation [91]. Knickelbein et al. reported that exosomes released by both resting and cytokine-stimulated RPE cells suppressed the proliferation of T lymphocytes isolated from the peripheral blood of noninfectious uveitis patients, and these nanosized vesicles could also regulate human monocyte phenotype and viability [59]. The above results indicate that exosome secretion may be a crucial mechanism for RPE cells to perform their immunoregulatory effects. Further understanding of exosomes from RPE cells may reveal novel vistas for therapy of uveitis.
Interestingly, Shigemoto-Kuroda and colleagues found that human bone marrow-derived MSC-Exos could effectively ameliorate experimental autoimmune uveoretinitis (EAU). The mixed lymphocyte reaction assay indicated that these MSC-Exos performed a significant inhibitory effect on the T cell proliferation and Th1 and Th17 development [60]. However, in another experimental study focused on EAU, human umbilical cord-derived MSC-Exos (hUC-MSC-Exos) failed to suppress the proliferation of conA-stimulated T cells, but effectively inhibited inflammatory cell migration [61]. In vitro results from our group showed that hUC-MSC-Exos had only a slight suppressive effect on interphotoreceptor retinoid-binding protein (IRBP)-specific Th17 responses, while they significantly inhibited DC-driven Th17 responses through the modulation of DC-derived Th17-polarizing cytokines IL-1β, IL-6, and IL-23. The discrepancies of these results may be due to the high heterogeneity of exosomes and distinct assay systems applied in the studies. It thus appears that MSC-Exos have therapeutic potential for autoimmune uveitis, but the specific mechanism related to their anti-inflammatory and immunomodulatory effects warrants further investigations.
Age-related macular degeneration (AMD)
Age-related macular degeneration (AMD), a complex multifactorial degenerative disease, is a leading cause of blindness among the elderly in developed countries [92]. Two clinical phenotypes of AMD exist: early non-exudative (dry-type) and late exudative (wet-type). The dry-type AMD is characterized by yellowish drusen (accumulation of extracellular deposits) and geographic atrophy, whereas the wet-type involves choroidal neovascularization (CNV) [93].
Gradually, it has been realized that pathological processes in AMD which had once been considered to be purely degenerative also implicate immune and inflammatory elements [21]. The complement system, a major arm of the innate immunity, has been recognized as a key component in AMD pathogenesis [94]. Reportedly, reduced membrane complement regulators in RPE cells contributed to RPE damage in AMD, and the decreased levels were partially explained by their release in apoptotic particles and exosomes [95]. Single nucleotide polymorphisms (SNPs) in complement factor H (CFH) gene have been identified to be linked with an increased risk of developing AMD [96, 97]. The CFH gene encodes protein factor H (FH) which functions as a regulator of the complement pathway [96]. Taylor et al. recently proposed that haploinsufficiency of factor H-like 1 (FHL-1), a variant of FH serving as a major complement regulator in Bruch’s membrane, may be an important mechanism driving the development of early-onset macular drusen in the vast majority of AMD cases [98]. Also, loss of complement protein C3 functionality contributes to the pathogenesis of AMD [99]. Dysfunction of CFH may cause C3-coated exosomes from RPE cells to become attacked by the invading leukocytes in the aged retina, and this might cause destabilization of exosome membranes and then result in the release of intracellular proteins, contributing to the formation of drusen [62]. These imply that RPE cell-derived exosomes are in part responsible for complement-driven innate immune responses in AMD.
In exudative AMD, especially in the CNV membranes, macrophages are the major populations of infiltrating inflammatory cells [100]. A pathological switch of macrophage polarization may be implicated in the development of CNV [101]. Retinal astrocyte-derived exosomes were confirmed to target both macrophages and vascular endothelial cells and perform significant inhibitory effects on laser-induced retinal vessel leakage and CNV of mouse models [63]. Besides, vascular endothelial growth factor (VEGF) has been identified as a critical inducer of pathologic neovascularization [102]. It is known that MSC-Exos are capable of regulating macrophage polarization [64] and downregulating VEGF expression [65]. Thereout, it can be speculated that MSC-Exos have the potential to control aberrant neovascularization in exudative AMD.
Exosome biomarkers for eye diseases
Exosomes and other EVs, particularly their cargoes, have been increasingly recognized as ideal low-invasive biomarkers in detecting, monitoring, and prognosticating diseases in recent years [103]. Especially in cancer screening, thermophoretic aptasensor has been developed to profile surface proteins of serum EVs for early cancer detection and classification [104]. Exosomes are abundant in tear fluids [105], aqueous humor (AH) [106], vitreous humor (VH) [107], and blood [108], all of which are important body fluids associated with ocular health and disease. Though it is less developed, theoretically, the identification and characterization of exosome-specific biomarkers in eye diseases have a great significance. For example, exosomes and their miRNA payload or proteomic profiling in AH may be used as novel diagnostic biomarkers for patients with glaucoma and neovascular AMD [106, 109]. Proteomic findings of RPE-derived exosomes may also offer diagnostic indicators for retinal disease [110]. Furthermore, Ragusa and colleagues showed that miR-146a was significantly upregulated in the VH exosomes of uveal melanoma patients with respect to controls, and the upregulation was also detected in serum exosomes of the same patients. Based on this, exosome-derived miR-146a might be deemed as a potential marker of uveal melanoma [107]. Overall, with the recent progress in exosome-specific isolation techniques and identification methods for their protein and nucleic acid contents, the research of exosome biomarkers for eye diseases appears to have sufficiently hopeful prospects.
Exosomes as drug delivery vesicles
The conventional route of treatment for eye disease, especially involving the anterior segment, is topical instillation of eye drops, which is accompanied with limitations such as the need for frequent administration and low bioavailability. During recent years, various synthetic drug vehicles have been developed for encasing existing drugs to enhance the therapeutic effect [111]. However, troubling issues including their immunotoxicity [112] and quick clearance by the mononuclear phagocyte system (MPS) or the reticuloendothelial system (RES) [113] still exist. Fortunately, exosomes, regarded as natural nanocarriers, have plenty of the highly desired qualities that drug delivery vehicles should have. These small vesicles are capable of penetrating the blood-brain barrier (BBB), delivering their cargoes across cell membranes and targeting specific cell types after artificial modifications [114]. Collectively, exosomes have been shown to serve as possible nanocarriers for functional RNA strands (mRNA, miRNA, siRNA, and lncRNA), DNA molecules, peptides, or synthetic drugs [115, 116]. For instance, exosomes from adeno-associated virus type 2 (AAV-2)-producing 293 T cells showed higher efficiency in retinal transduction than conventional AAV-2 after intravitreal injection and were regarded as robust tools for intravitreal gene transfer into the retina [117]. Besides, MSC-Exos loaded with exogenous miRNA-126 were reported to alleviate hyperglycemia-induced retinal inflammation via suppressing the high-mobility group box 1 (HMGB1) signal pathway [66]. Moreover, chemotherapeutic drug-loaded exosomes showed higher efficacy and better bioavailability compared to free drug [118, 119], which sheds new light on ocular pharmacotherapeutics. So far, there has been sparse research focused on the latent role of loading exosomes with exogenous functional cargoes in eye diseases. Therefore, significant endeavors are needed to develop such therapies in ophthalmology.
Conclusions
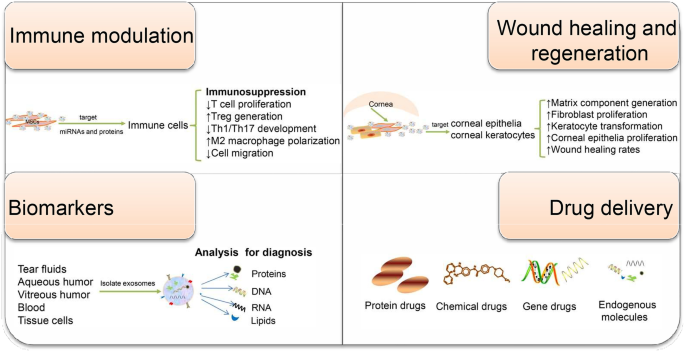
Taken together, the extensive implication of exosomes in regulating various aspects of the immunity makes exosomes attractive diagnostic and therapeutic candidates for immune-mediated eye diseases (Fig. 2). Because of their multiple functions, elucidating the contents of exosomes and understanding how each of them function are necessary. Additionally, for successful translation into clinical therapies, novel and advanced technology is urgently needed to obtain mass highly purified exosomes with stable functional efficacy. Exosome research in the eye is still a relatively young field, awaiting more extensive investigations into the precise biological mechanisms and clinical potential of exosomes in ocular diseases.
Schematic of the potential application of exosomes in immune-mediated eye diseases. Exosomes have been involved in a broad variety of physiological and pathophysiological events. Depending on their origin and exposure context, they exert different functions including intercellular communication, immune modulation, wound healing, and regeneration. MSC-Exos have been found to regulate the activity of intraocular immune cells. Corneal epithelial cell-derived exosomes are capable of promoting matrix component generation, and corneal limbal keratocyte-derived exosomes can accelerate corneal wound healing. Moreover, exosomal miRNA payload or proteomic profiling can reflect the disease state and have the potential to serve as eye disease-specific biomarkers. Owing to their highly desired drug carrier attributes, exosomes are increasingly considered as ideal drug delivery systems. Together, exosome-based therapy or diagnosis holds great potential for clinical application in ophthalmology
Availability of data and materials
Not applicable.
Abbreviations
- AAV-2:
-
Adeno-associated virus type 2
- AH:
-
Aqueous humor
- AMD:
-
Age-related macular degeneration
- AQP5:
-
Aquaporin 5
- BAFF:
-
B cell activating factor
- BBB:
-
Blood-brain barrier
- CFH:
-
Complement factor H
- CNV:
-
Choroidal neovascularization
- DCs:
-
Dendritic cells
- EAU:
-
Experimental autoimmune uveoretinitis
- EBV:
-
Epstein-Barr virus
- ESCRT:
-
Endosomal sorting complex required for transport
- EVs:
-
Extracellular vesicles
- FH:
-
Factor H
- FHL-1:
-
Factor H like 1
- HMGB1:
-
High-mobility group box 1
- hUC-MSC-Exos:
-
Human umbilical cord-derived MSC-Exos
- IFN:
-
Interferon
- ILV:
-
Intraluminal vesicles
- iNOS:
-
Inducible nitric oxide synthase
- IRBP:
-
Interphotoreceptor retinoid-binding protein
- LGs:
-
Lacrimal glands
- MHC:
-
Major histocompatibility complex
- MPS:
-
Mononuclear phagocyte system
- MSC-Exos:
-
Mesenchymal stem cells-derived exosomes
- MSCs:
-
Mesenchymal stem cells
- MVBs:
-
Multivesicular bodies
- NK:
-
Natural killer
- PD-L1:
-
Programmed death-ligand 1
- RES:
-
Reticuloendothelial system
- RNPs:
-
Ribonucleoproteins
- RPE:
-
Retinal pigment epithelium
- SGECs:
-
Salivary gland epithelial cells
- SNARE:
-
Soluble N-ethylmaleimide-sensitive fusion attachment protein receptor
- SNPs:
-
Single nucleotide polymorphisms
- SS:
-
Sjögren’s syndrome
- STIM1:
-
Stromal interacting molecule 1
- TLRs:
-
Toll-like receptors
- Tregs:
-
Regulatory T cells
- TSG101:
-
Tumor susceptibility gene 101
- VEGF:
-
Vascular endothelial growth factor
- VH:
-
Vitreous humor
References
- 1.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8.
- 2.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78.
- 3.
Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA, et al. Biological functions and current advances in isolation and detection strategies for exosome nanovesicles. Small. 2018;14(6).
- 4.
Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017;31(6):1259–68.
- 5.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
- 6.
Klingeborn M, Dismuke WM, Bowes Rickman C, Stamer WD. Roles of exosomes in the normal and diseased eye. Prog Retin Eye Res. 2017;59:158–77.
- 7.
Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46(D1):D106–d12.
- 8.
Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–9.
- 9.
Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41.
- 10.
Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. 2019;18(1):57.
- 11.
Wang H, Cheng X, Li M, Li W, Zhu T, Li Q. Adaptive immune stimuli altered the cargo proteins of exosomes released by supraneural myeloid body cells in Lampetra japonica. Mol Immunol. 2019;111:64–72.
- 12.
Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18(1):59.
- 13.
Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–23.
- 14.
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4).
- 15.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208.
- 16.
Anel A, Gallego-Lleyda A, de Miguel D, Naval J, Martinez-Lostao L. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells. 2019;8((2).
- 17.
Xu H, Jia S, Xu H. Therapeutic potential of exosomes in autoimmune diseases. Clin Immunol. 2019.
- 18.
Keino H, Horie S, Sugita S. Immune privilege and eye-derived T-regulatory cells. J Immunol Res. 2018;2018:1679197.
- 19.
Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R. Management of high-risk corneal transplantation. Surv Ophthalmol. 2017;62(6):816–27.
- 20.
Bose T, Diedrichs-Möhring M, Wildner G. Dry eye disease and uveitis: a closer look at immune mechanisms in animal models of two ocular autoimmune diseases. Autoimmun Rev. 2016;15(12):1181–92.
- 21.
Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36(6):354–63.
- 22.
Ratay ML, Bellotti E, Gottardi R, Little SR. Modern therapeutic approaches for noninfectious ocular diseases involving inflammation. Adv Healthcare Mater. 2017;6(23).
- 23.
de Andrade FA, Fiorot SH, Benchimol EI, Provenzano J, Martins VJ, Levy RA. The autoimmune diseases of the eyes. Autoimmun Rev. 2016;15(3):258–71.
- 24.
Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
- 25.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
- 26.
Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):32.
- 27.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
- 28.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3.
- 29.
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7.
- 30.
Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76(9):1747–58.
- 31.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
- 32.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, et al. B lymphocytes secrete antigen-presenting vesicles. 1996;183(3):1161–72.
- 33.
Ren K. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology. 2018.
- 34.
Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81.
- 35.
Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insights Pathol. 2016;9(Suppl 1):1–8.
- 36.
Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103.
- 37.
Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26(19):4263–72.
- 38.
Nolte-‘t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–81.
- 39.
Buschow SI, Nolte-‘t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–42.
- 40.
Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14(7):713–22.
- 41.
Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27 e13.
- 42.
Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019:5126156.
- 43.
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019.
- 44.
Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, et al. Exosomes originating from MSCs stimulated with TGF-beta and IFN-gamma promote Treg differentiation. J Cell Physiol. 2018;233(9):6832–40.
- 45.
Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36(1):79–90.
- 46.
Domenis R, Cifu A, Quaglia S, Pistis C, Moretti M, Vicario A, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8(1):13325.
- 47.
Barros FM, Carneiro F, Machado JC, Melo SA. Exosomes and immune response in cancer: friends or foes? Front Immunol. 2018;9:730.
- 48.
Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, et al. Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 2017;20(1):99–111.
- 49.
Chan BD, Wong WY, Lee MM, Cho WC, Yee BK, Kwan YW, et al. Exosomes in inflammation and inflammatory disease. Proteomics. 2019:e1800149.
- 50.
Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16(1):143.
- 51.
Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52(5):1517–21.
- 52.
Gallo A, Jang SI, Ong HL, Perez P, Tandon M, Ambudkar I, et al. Targeting the Ca(2+) sensor STIM1 by exosomal transfer of Ebv-miR-BART13-3p is associated with Sjogren’s syndrome. EBioMedicine. 2016;10:216–26.
- 53.
Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L, et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci Rep. 2017;7(1):11518.
- 54.
Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166–75.
- 55.
Jangamreddy JR, Haagdorens MKC, Mirazul Islam M, Lewis P, Samanta A, Fagerholm P, et al. Short peptide analogs as alternatives to collagen in pro-regenerative corneal implants. Acta Biomater. 2018;69:120–30.
- 56.
Han KY, Tran JA, Chang JH, Azar DT, Zieske JD. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci Rep. 2017;7:40548.
- 57.
Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep. 2018;8(1):15173.
- 58.
Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, et al. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2018;59(12):5194–200.
- 59.
Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, et al. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2016;57(10):4101–7.
- 60.
Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8(5):1214–25.
- 61.
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7(1):4323.
- 62.
Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4(1):e4160.
- 63.
Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, et al. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem. 2013;288(39):28058–67.
- 64.
Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(−/−) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–72.
- 65.
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017;40(5):457–70.
- 66.
Zhang W, Wang Y, Kong Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60(1):294–303.
- 67.
Shiboski SC, Shiboski CH, Criswell LA, Baer AN, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64(4):475–87.
- 68.
Lopez-Miguel A, Teson M, Martin-Montanez V, Enriquez-de-Salamanca A, Stern ME, Gonzalez-Garcia MJ, et al. Clinical and molecular inflammatory response in Sjogren syndrome-associated dry eye patients under desiccating stress. Am J Ophthalmol. 2016;161:133–41 e1–2.
- 69.
Sandhya P, Kurien BT, Danda D, Scofield RH. Update on pathogenesis of Sjogren’s syndrome. Curr Rheumatol Rev. 2017;13(1):5–22.
- 70.
Mavragani CP. Mechanisms and new strategies for primary Sjogren’s syndrome. Annu Rev Med. 2017;68:331–43.
- 71.
Manoussakis MN, Kapsogeorgou EK. The role of intrinsic epithelial activation in the pathogenesis of Sjogren’s syndrome. J Autoimmun. 2010;35(3):219–24.
- 72.
Generali E, Costanzo A, Mainetti C, Selmi C. Cutaneous and mucosal manifestations of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2017;53(3):357–70.
- 73.
Goules AV, Kapsogeorgou EK, Tzioufas AG. Insight into pathogenesis of Sjogren’s syndrome: dissection on autoimmune infiltrates and epithelial cells. Clin Immunol. 2017;182:30–40.
- 74.
Mavragani CP, Moutsopoulos HM. Sjogren’s syndrome. Annu Rev Pathol. 2014;9:273–85.
- 75.
Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES192–DES9.
- 76.
Li X, Lu X, Sun D, Wang X, Yang L, Zhao S, et al. Adipose-derived mesenchymal stem cells reduce lymphocytic infiltration in a rabbit model of induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2016;57(13):5161–70.
- 77.
Satitpitakul V, Sun Z, Suri K, Amouzegar A, Katikireddy KR, Jurkunas UV, et al. Vasoactive intestinal peptide promotes corneal allograft survival. Am J Pathol. 2018;188(9):2016–24.
- 78.
Tariq M, Havens SJ. Corneal graft rejection. StatPearls. Treasure Island: StatPearls Publishing StatPearls Publishing LLC; 2018.
- 79.
Marino J, Paster J, Benichou G. Allorecognition by T lymphocytes and allograft rejection. Front Immunol. 2016;7:582.
- 80.
Gonzalez-Nolasco B, Wang M, Prunevieille A, Benichou G. Emerging role of exosomes in allorecognition and allograft rejection. Curr Opin Organ Transplant. 2018;23(1):22–7.
- 81.
Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–73.
- 82.
Williams R, Lace R, Kennedy S, Doherty K, Levis H. Biomaterials for regenerative medicine approaches for the anterior segment of the eye. Adv Healthcare Mater. 2018;7(10):e1701328.
- 83.
Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther. 2016;16(4):489–506.
- 84.
Papotto PH, Marengo EB, Sardinha LR, Goldberg AC, Rizzo LV. Immunotherapeutic strategies in autoimmune uveitis. Autoimmun Rev. 2014;13(9):909–16.
- 85.
Krishna U, Ajanaku D, Denniston AK, Gkika T. Uveitis: a sight-threatening disease which can impact all systems. Postgrad Med J. 2017;93(1106):766–73.
- 86.
Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43(6):1040–51.
- 87.
Pepple KL, Lin P. Targeting Interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125(12):1977–83.
- 88.
Chong WP, van Panhuys N, Chen J, Silver PB, Jittayasothorn Y, Mattapallil MJ, et al. NK-DC crosstalk controls the autopathogenic Th17 response through an innate IFN-gamma-IL-27 axis. J Exp Med. 2015;212(10):1739–52.
- 89.
Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–83.
- 90.
Sevgi DD, Davoudi S, Comander J, Sobrin L. Retinal pigmentary changes in chronic uveitis mimicking retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1801–10.
- 91.
Shi Q, Wang Q, Li J, Zhou X, Fan H, Wang F, et al. A2E suppresses regulatory function of RPE cells in Th1 cell differentiation via production of IL-1beta and inhibition of PGE2. Invest Ophthalmol Vis Sci. 2015;56(13):7728–38.
- 92.
Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e16.
- 93.
Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3:34.
- 94.
Bora NS, Matta B, Lyzogubov VV, Bora PS. Relationship between the complement system, risk factors and prediction models in age-related macular degeneration. Mol Immunol. 2015;63(2):176–83.
- 95.
Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229(5):729–42.
- 96.
Liszewski MK, Java A, Schramm EC, Atkinson JP. Complement dysregulation and disease: insights from contemporary genetics. Annu Rev Pathol. 2017;12:25–52.
- 97.
Toomey CB, Johnson LV, Bowes RC. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57.
- 98.
Taylor RL, Poulter JA, Downes SM, McKibbin M, Khan KN, Inglehearn CF, et al. Loss-of-function mutations in the CFH gene affecting alternatively encoded factor H-like 1 protein cause dominant early-onset macular drusen. Ophthalmology. 2019.
- 99.
Park DH, Connor KM, Lambris JD. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10:1007.
- 100.
Nussenblatt RB, Liu B, Wei L, Sen HN. The immunological basis of degenerative diseases of the eye. Int Rev Immunol. 2013;32(1):97–112.
- 101.
Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan Z, et al. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci Rep. 2016;6:30933.
- 102.
Sene A, Chin-Yee D, Apte RS. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med. 2015;21(1):43–51.
- 103.
González E, Falcón-Pérez J. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev Mol Diagn. 2015;15(7):907–23.
- 104.
Liu C, Zhao J, Tian F, Cai L, Zhang W, Feng Q, et al. Low-cost thermophoretic profiling of extracellular-vesicle surface proteins for the early detection and classification of cancers. Nat Biomed Eng. 2019;3(3):183–93.
- 105.
Grigor’eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, et al. Characteristics of exosomes andmicroparticles discovered in human tears. Biomeditsinskaia khimiia. 2016;62(1):99–106.
- 106.
Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–7.
- 107.
Ragusa M, Barbagallo C, Statello L, Caltabiano R, Russo A, Puzzo L, et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: pathological and diagnostic implications. Cancer Biol Ther. 2015;16(9):1387–96.
- 108.
Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67(8):1639–49.
- 109.
Kang GY, Bang JY, Choi AJ, Yoon J, Lee WC, Choi S, et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J Proteome Res. 2014;13(2):581–95.
- 110.
Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes RC. Directional exosome proteomes reflect polarity-specific functions in retinal pigmented epithelium monolayers. Sci Rep. 2017;7(1):4901.
- 111.
Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine (London). 2017;12(6):683–702.
- 112.
Pandey RK, Prajapati VK. Molecular and immunological toxic effects of nanoparticles. Int J Biol Macromol. 2018;107(Pt A):1278–93.
- 113.
Haque S, Whittaker MR, McIntosh MP, Pouton CW, Kaminskas LM. Disposition and safety of inhaled biodegradable nanomedicines: opportunities and challenges. Nanomedicine. 2016;12(6):1703–24.
- 114.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405.
- 115.
Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78.
- 116.
Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65.
- 117.
Wassmer SJ, Carvalho LS, Gyorgy B, Vandenberghe LH, Maguire CA. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci Rep. 2017;7:45329.
- 118.
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–79.
- 119.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (81770901, 81570834), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 48), and the Tianjin Clinical Key Discipline Project (TJLCZDXKT003). The authors would like to thank Tianjin Medical University Eye Hospital, Eye Institute, for its supports in developing this paper.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770901, 81570834), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 48), and the Tianjin Clinical Key Discipline Project (TJLCZDXKT003).
Author information
Affiliations
Contributions
NL read the literature related to the topic and participated in drafting the manuscript. LZ and YW participated in searching and archived the literature related to the topic and discussed the contents of the manuscript. VE revised the manuscript. HN and RW participated in the design, revision, and final approval of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Na Li, Postgraduate, is studying in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. Lu Zhao, Postgraduate, is studying in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. Yankai Wei, Ph.D., candidate, is studying in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. Vicki L. Ea, Researcher, is working in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. Her research focus was on diabetic retinopathy. Hong Nian, PhD, is a Principal Investigator in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. She focused on studying the mechanisms of autoimmune diseases, including autoimmune dacryoadenitis and autoimmune uveitis. Ruihua Wei, MD, Chief Consultant, is working in Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, No. 251, Fukang Road, Nankai District, Tianjin 300384, China. She is engaged in the diagnosis and treatment of ocular surface diseases and myopia.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Molecular Vision 2016; 22:1095-1102