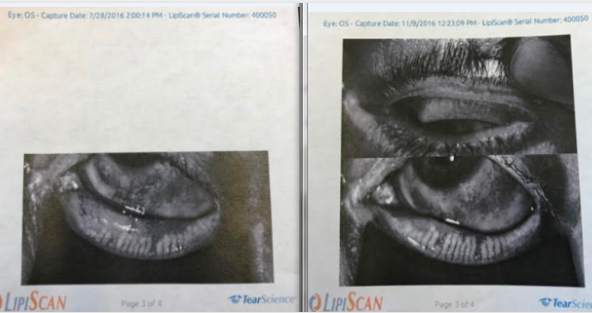
One of the best things about injecting stem cells into the Meibomian Glands is that we have an objective way to see if it works or not: Meibography or LipiScan.
I had posted before about the before and after photos of a patient who had IPL: Intense Pulse Light
https://drcremers.com/2016/11/ipl-intense-pulse-light-before-and.html?q=2+patients
Left Lower Lid Before IPL on the left photo. After IPL of left lower lid on right photo.
The study below used a rat’s bone marrow to obtain stem cells. I suspect if they had used the rat’s fat, they might have had even better results.
I suspect fat derived stem cells may be an alternative option to autolgous serum and may help those patients who do not improve on autologous serum alone: many have Chronic Neurotropic Dry Eye Disease from Lasik (CiNDryELa). Some of the these patients who have had no benefit with Autologous Serum have underlying autoimmune disease.
Sandra Lora Crermers, MD, FACS
Stem Cells International Volume 2014 (2014), Article ID 250230, 9 pages http://dx.doi.org/10.1155/2014/250230
Efficacy of Topical Mesenchymal Stem Cell Therapy in the Treatment of Experimental Dry Eye Syndrome Model
Purpose. The current study was set out to address the therapeutic efficacy of topically applied mesenchymal stem cells (MSCs) on dry eye syndrome (DES) induced by benzalkonium chloride (BAC) in rats. Methods. Rats were divided into two groups just after establishment of DES. Eye drops containing either bromodeoxyuridine labeled MSCs () or phosphate buffer solution () were topically applied once daily for one week. Schirmer test, break-up time score, ocular surface evaluation tests, and corneal inflammatory index scoring tests were applied to all rats at baseline and after treatment. All rats were sacrificed after one week for histological and electron microscopic analysis. Results. Mean aqueous tear volume and tear film stability were significantly increased in rats treated with MSCs (). Infiltration of bromodeoxyuridine labeled MSCs into the meibomian glands and conjunctival epithelium was observed in MSCs treated rats. Increased number of secretory granules and number of goblet cells were observed in MSCs treated rats. Conclusion. Topical application of MSCs could be a safe and effective method for the treatment of DES and could potentially be used for further clinical research studies.

