One of the most common complaints we encounter in the clinical setting is ocular discomfort, typically in the form of dryness, irritation and foreign body sensation. These annoying symptoms are among the most pervasive and vague. While dry eye syndrome is an exceedingly prevalent diagnosis in our adult population, we must differentiate aqueous deficiency or evaporative dry eye from more complex and, potentially, refractory conditions. Filamentary keratitis is one such condition.
In this column, we’ll discuss the key diagnosing filamentary keratitis, as well as the various treatment modalities for controlling it.
At-Risk Patients While the exact prevalence of filamentary keratitis is unknown, experience suggests it’s more common in elderly patients, females and those with connective tissue disorders or immune deficiency.1,2 The exact nature and severity of symptoms ranges from mild ocular discomfort to pronounced pain. Tearing, photophobia and even blepharospasm may accompany these symptoms in severe cases.3
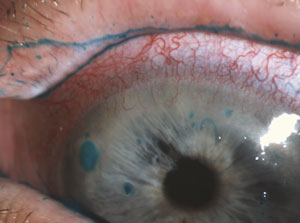
Associated Signs Signs associated with filamentary keratitis include ocular hyperemia, particularly in the limbal area, as well as a pseudoptosis in some individuals. Corneo-mucus filaments are the hallmark finding. These usually consist of a focal “head,” which may
|
|
| Corneal filaments stained with lissamine green are evident in this red, inflamed eye. |
|
firmly adhere to compromised areas of the corneal epithelium, and a strand-like “tail” of varying length that extends across the ocular surface. Applying vital dyes, such as lissamine green, rose bengal and sodium fluorescein, can aid biomicroscopic filament visualization.3 A rapid tear break-up time and punctate epithelial keratopathy may also be present.
Not only does filamentary keratitis accompany dry eye, it also appears alongside a variety of other ocular surface disorders, including superior limbic keratoconjunctivitis, prolonged patching following ocular surgery, epitheliopathy due to aerosol or radiation keratitis, herpetic keratitis, recurrent corneal erosion, neurotrophic keratitis and bullous keratopathy.1-5
Mechanism Research suggests that individual filaments consist of desquamated corneal epithelial cells (at their cores), surrounded primarily by degenerating conjunctival epithelial cells and entwined in a thick layer of membrane-associated mucins.4,5 Patients with filamentary keratitis appear to suffer progressive dysfunction within the basal epithelial and Bowman’s layers of the cornea, leading to focal detachments at the level of the basement membrane. Under constant shear pressure from the eyelids, these corneal foci become inflamed, and sloughing of epithelial cells may ensue.6 At the same time, frictional stress from blinking and eye movement combined with diminished tear volume and ocular surface inflammation results in abnormal tear mucin production and degeneration of conjunctival epithelial cells.5
These combined elements form filaments, which may appear clinically as long strands, large clumps or irregular dendriform deposits, depending upon whether they are stretched, twisted or tightly coiled.4,7 The filaments are motile in the tear film, but have an affinity for compromised areas of the corneal surface, where they form strong adhesions. Lid movement across these bound filaments induces vertical traction and further shearing of the corneal epithelium with each blink, resulting in microtrauma and stimulation of the pain-sensitive corneal nerves. Thus, a vicious cycle of epithelial damage, inflammation and filament formation ensues.
Management The management of filamentary keratitis is aimed at alleviating the stressors that cause ocular surface inflammation and epithelial degradation. Elimination of the filaments is the initial step, but identifying and treating the underlying pathology is vital to breaking the cycle of this disease. You can remove large filaments mechanically using fine-tipped forceps at the slit lamp under topical anesthesia. Recognize, however, that this process can further contribute to epithelial damage and should be undertaken only by skilled and experienced clinicians. Ocular lubricants are helpful in addressing discomfort and also stabilizing the tear film in mild to moderate cases.
In recalcitrant cases, topical N-acetylcysteine can help to dissolve cornea-bound mucus plaques.1 This mucolytic agent is employed primarily as an oral inhalant for patients with bronchial disease (e.g. emphysema, cystic fibrosis), and hence it must be prepared by a compounding pharmacist for topical ophthalmic use. In those with filamentary keratitis secondary to chronic dry eye disease, we have seen excellent results with 10% acetylcysteine eye drops used four times daily for several weeks. Other treatments for refractory cases of filamentary keratitis may include the use of bandage soft contact lenses, amniotic membrane therapy or Botox (onabotulinumtoxinA, Allergan) injection to the pretarsal orbicularis muscle.2,8
Long-term Treatment Addressing the underlying ocular surface disease may ultimately prove more challenging than temporary elimination of corneal filaments. Because an inflammatory etiology is often assumed, the use of anti-inflammatory drugs such as corticosteroids and non-steroidal agents has been widely advocated, often with clinical success.9,10 In those cases where dry eye disease is identified as the primary etiology of filamentary keratitis, short-term use of corticosteroids such asLotemax (loteprednol etabonate 0.5%, Bausch + Lomb) QID combined with long-term use of Restasis (cyclosporine, Allergan) BID can help.11
Severe cases may require treatment with autologous serum eye drops, which—as the name implies—are derived from the patient’s own blood serum.12,13
Therapy for filamentary keratitis may take weeks or even months before adequate resolution is realized, depending on the etiology, severity of presentation and aggressiveness of care. Patients should understand that the underlying condition is often chronic and filaments may recur after therapy is discontinued. Proper long-term care includes ongoing treatment for ocular surface disease with close monitoring, i.e., three to four times annually. In addition, patients with chronic or severe dry eye disease may benefit from a rheumatologic investigation to determine the presence of Sjögren’s syndrome.14
Dr. Kabat is a consultant to Alcon Laboratories, Bio-Tissue and BlephEx. Neither he nor Dr. Sowka has any direct financial interest in the products mentioned in this article.
1. Albietz J, Sanfilippo P, Troutbeck R, Lenton LM. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci. 2003 Jun;80(6):420-30. 2. Gumus K, Lee S, Yen MT, Pflugfelder SC. Botulinum toxin injection for the management of refractory filamentary keratitis. Arch Ophthalmol. 2012 Apr;130(4):446-50. 3. Diller R, Sant S. A case report and review of filamentary keratitis. Optometry. 2005 Jan;76(1):30-6. 4. Tabery HM. Filamentary keratopathy: a non-contact photomicrographic in vivo study in the human cornea. Eur J Ophthalmol. 2003 Aug-Sep;13(7):599-605. 5. Tanioka H, Yokoi N, Komuro A, et al. Investigation of the corneal filament in filamentary keratitis. Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3696-702. 6. Zaidman GW, Geeraets R, Paylor RR, Ferry AP. The histopathology of filamentary keratitis. Arch Ophthalmol. 1985 Aug;103(8):1178-81. 7. Pandit RT. Dendriform filamentary keratopathy. Cornea. 2009 Jan;28(1):123-5. 8. Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013 Sep;39(5):341-7. 9. Perry HD, Doshi-Carnevale S, Donnenfeld ED, Kornstein HS. Topical cyclosporine A 0.5% as a possible new treatment for superior limbic keratoconjunctivitis. Ophthalmology. 2003 Aug;110(8):1578-81. 10. Terry G Coursey, Cintia S de Paiva. Managing Sjögren’s Syndrome and non-Sjögren Syndrome dry eye with anti-inflammatory therapy. Clin Ophthalmol. 2014; 8: 1447–1458. 11. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014 Sep;40(5):289-96. 12. Jirsova K, Brejchova K, Krabcova I, et al. The application of autologous serum eye drops in severe dry eye patients; subjective and objective parameters before and after treatment. Curr Eye Res. 2014 Jan;39(1):21-30. 13. Hussain M, Shtein RM, Sugar A, et al. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. Cornea. 2014 Dec;33(12):1245-51. 14. Shen L, Kapsogeorgou EK, Yu M, et al. Evaluation of salivary gland protein 1 antibodies in patients with primary and secondary Sjogren’s syndrome. Clin Immunol. 2014 Nov;155(1):42-6.







