Here is a great summary of what to be aware of with hormone levels in general and dry eyes.
SLC
Hormones and Dry Eye Syndrome
An Update on What We Do and Don’t Know
- Abstract and Introduction
- Sex Hormones
- Thyroid Hormone-related Diseases and Dry Eye Syndrome
- Diabetic Dry Eye
- Gene Therapy for LG
- Conclusion
Abstract
Purpose of review Dry eye syndrome (DES) prevalence is large and its relationship with hormonal diseases is becoming clearer, although more complex. This review provides insight to this association as well as clarifying what remains unanswered about how to interpret and treat findings common to both DES and hormonal diseases.
Recent findings Several sex hormone-related diseases are associated with DES. Hormone replacement therapy to correct such conditions has conflicting outcomes based on epidemiologic studies and clinical trials. Thyroid-associated diseases are frequently involved in DES and must be investigated in cases where the cause of the ocular disease is undetermined. Diabetes mellitus is one of the major causes of DES, whereas correcting the metabolic imbalance minimizes its ocular symptomology. Gene therapy to treat DES-related hormonal diseases is a promising option based on animal studies.
Summary Diagnosis and management of hormonal diseases can minimize the ocular surface damage and severity of DES. Clinical care of DES includes patient evaluation of hormonal status. Future research requires clarification of the underlying disease mechanisms and identifying novel strategies to reprogram the endocrine system rather than chronic medication usage.
Introduction
As early as 1930, Dr Henrik Sjögren indicated that there is an association between hormonal changes and dry eye syndrome (DES).[1] His first study mentioned the disease only in women and had one patient with glucose tolerance test results reflective of an insulin resistance. Since then, numerous other results confirmed an association between endocrine system balance, ocular surface homeostasis and lacrimal gland function.[2] Moreover, the role of hormonal diseases in DES progression is now more extensively described providing novel perspectives for hormonal treatment of this condition.
Currently, it is apparent that in several different diseases caused by a hormonal imbalance, DES clinical findings occur (Table 1).
This review analyzes recent controversy surrounding the relationship among menopause changes and DES. It focuses also on the association between DES and the most common endocrine diseases involving changes in sex hormones, thyroid-associated diseases, and diabetes mellitus. A complementary topic describes the potential use of gene therapy to reprogram lacrimal gland function so as to overcome hormonal disease-induced ocular surface damage.
Sex Hormones
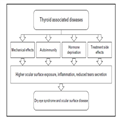
Thyroid Hormone-related Diseases and Dry Eye Syndrome
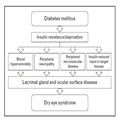
Diabetic Dry Eye
Gene Therapy for LG
Conclusion
References
-
Murube J. Henrik Sjögren, 1899–1986. Ocul Surf 2010; 8:2–7.
-
Sullivan DA, Wickham LA, Rocha EM, et al. Influence of gender, sex steroid hormones, and the hypothalamic-pituitary axis on the structure and function of the lacrimal gland. Adv Exp Med Biol 1998; 438:11–42.
-
Mantelli F, Moretti C, Micera A, Bonini S. Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS). Graefes Arch Clin Exp Ophthalmol 2007; 245:899–902.
-
Sullivan BD, Evans JE, Cermak JM, et al. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol 2002; 120:1689–1699.
-
Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab 2000; 85:4874–4882.
-
Coksuer H, Ozcura F, Oghan F, et al. Effects of hyperandrogenism on tear function and tear drainage in patients with polycystic ovary syndrome. J Reprod Med 2011; 56:65–70.
-
Bonini S, Mantelli F, Moretti C, et al. Itchy-dry eye associated with polycystic ovary syndrome. Am J Ophthalmol 2007; 143:763–771.
-
Alves M, Dias AC, Rocha EM. Dry eye in childhood: epidemiological and clinical aspects. Ocul Surf 2008; 6:44–51.
-
Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. J Am Med Assoc 2001; 286:2114–2119.
-
Jensen AA, Higginbotham EJ, Guzinski GM, et al. A survey of ocular complaints in postmenopausal women. J Assoc Acad Minor Phys 2000; 11:44–49.
-
Okon A, Jurowski P, Gos R. The influence of the hormonal replacement therapy on conjunctival epithelium morphology among peri- and postmenopausal women. Klinika oczna 2001; 103:183–186.
-
Chia EM, Mitchell P, Rochtchina E, et al. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol 2003; 31:229–232.
-
Nagler RM, Pollack S. Sjögren’s syndrome induced by estrogen therapy. Semin Arthritis Rheum 2000; 30:209–214.
-
Erdem U, Ozdegirmenci O, Sobaci E, et al. Dry eye in postmenopausal women using hormone replacement therapy. Maturitas 2007; 56:257–262.
-
Uncu G, Avci R, Uncu Y, et al. The effects of different hormone replacement therapy regimens on tear function, intraocular pressure and lens opacity. Gynecol Endocrinol 2006; 22:501–505.
-
Mathers WD, Stovall D, Lane JA, et al. Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea 1998; 17:353–358.
-
Wenderlein M, Mattes S. The ‘dry eye’ phenomenon and ovarian function. Study of 700 women pre and postmenopausal. Zentralblatt fur Gynakologie 1996; 118:643–649.
-
Coksuer H, Ozcura F, Oghan F, et al. Effects of estradiol-drospirenone on ocular and nasal functions in postmenopausal women. Climacteric 2011; 14:482–487.
-
Sullivan DA, Belanger A, Cermak JM, et al. Are women with Sjögren’s syndrome androgen-deficient? J Rheumatol 2003; 30:2413–2419.
-
Haga HJ, Gjesdal CG, Irgens LM, Ostensen M. Reproduction and gynaecological manifestations in women with primary Sjögren’s syndrome: a case-control study. Scand J Rheumatol 2005; 34:45–48.
-
Nielsen NM, Jorgensen KT, Pedersen BV, et al. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjögren syndrome. Hum Reprod 2011; 26:1555–1559.
-
Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Molec Vis 2009; 15:1553–1572.
-
Porola P, Straub RH, Virkki LM, et al. Failure of oral DHEA treatment to increase local salivary androgen outputs of female patients with Sjögren’s syndrome. Scand J Rheumatol 2011; 40:387–390.
-
Labrie F, Labrie C. DHEA and intracrinology at menopause, a positive choice for evolution of the human species. Climacteric 2013; 16:205–213.
* This review explains the intracrine events related to DHEA in postmenopausal women, suggesting that it is a positive evolutionary process.
-
Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol 2011; 152:377–384.e2.
-
Coll J, Anglada J, Tomas S, et al. High prevalence of subclinical Sjögren’s syndrome features in patients with autoimmune thyroid disease. J Rheumatol 1997; 24:1719–1724.
-
Mergler S, Garreis F, Sahlmuller M, et al. Thermosensitive transient receptor potential channels in human corneal epithelial cells. J Cell Physiol 2011; 226:1828–1842.
-
Pan Z, Wang Z, Yang H, et al. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2011; 52:485–493.
-
Yang H, Wang Z, Capo-Aponte JE, et al. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp Eye Res 2010; 91:462–471.
-
Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum Genomics 2011; 5:108–116.
-
Garcia DM, Barbosa JC, Pinto CT, Cruz AA. Estimation of spontaneous blinking main sequence in normal subjects and patients with Graves’ upper eyelid retraction. Invest Ophthalmol Vis Sci 2013; 54:1434–1442.
** This study compares the characteristics of blinking in Graves’ and control individuals.
-
Brasil MV, Brasil OF, Vieira RP, et al. Tear film analysis and its relation with palpebral fissure height and exophthalmos in Graves’ ophthalmopathy. Arq Bras Oftalmol 2005; 68:615–618.
-
Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol (Copenh) 1983; 61:108–116.
-
Biro E, Szekanecz Z, Czirjak L, et al. Association of systemic and thyroid autoimmune diseases. Clin Rheumatol 2006; 25:240–245.
-
Jara LJ, Navarro C, Brito-Zeron Mdel P, et al. Thyroid disease in Sjögren’s syndrome. Clin Rheumatol 2007; 26:1601–1606.
-
Harris MA, Realini T, Hogg JP, Sivak-Callcott JA. CT dimensions of the lacrimal gland in Graves orbitopathy. Ophthal Plast Reconstructr Surg 2012; 28:69–72.
* This study establishes correlation between the lacrimal gland morphology and a clinical disease using a noninvasive method.
-
Eckstein AK, Finkenrath A, Heiligenhaus A, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand 2004; 82 (3 Pt 1):291–297.
-
Ruggeri RM, Galletti M, Mandolfino MG, et al. Thyroid hormone autoantibodies in primary Sjögren syndrome and rheumatoid arthritis are more prevalent than in autoimmune thyroid disease, becoming progressively more frequent in these diseases. J Endocrinol Invest 2002; 25:447–454.
-
Dias AC, Modulo CM, Jorge AG, et al. Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest Ophthalmol Vis Sci 2007; 48:3038–3042.
-
Goolamali SK, Evered D, Shuster S. Thyroid disease and sebaceous function. Br Med J 1976; 1:432–433.
-
Gurdal C, Genc I, Sarac O, et al. Topical cyclosporine in thyroid orbitopathy-related dry eye: clinical findings, conjunctival epithelial apoptosis, and MMP-9 expression. Curr Eye Res 2010; 35:771–777.
-
Bartley GB, Fatourechi V, Kadrmas EF, et al. The treatment of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996; 121:200–206.
-
Bartley GB, Fatourechi V, Kadrmas EF, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology 1996; 103:958–962.
-
Prabhu RS, Liebman L, Wojno T, et al. Clinical outcomes of radiotherapy as initial local therapy for Graves’ ophthalmopathy and predictors of the need for postradiotherapy decompressive surgery. Radiat Oncol 2012; 7:95–102.
-
Matthiesen C, Thompson JS, Thompson D, et al. The efficacy of radiation therapy in the treatment of Graves’ orbitopathy. Int J Radiat Oncol Biol Phys 2012; 82:117–123.
* The study followed more than 200 patients along of 10 years and presented a rate of success of 84% of Graves’ ophthalmopathy treatment with radiotherapy. However, it also shows that 12% of patients developed DES.
-
Wang TJ, Wang IJ, Hu CC, Lin HC. Comorbidities of dry eye disease: a nationwide population-based study. Acta Ophthalmol 2012; 90:663–668.
** This is a national epidemiologic study conducted in Taiwan with more than 10 000 individuals. It identified the comorbidities of DES.
-
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997; 14 (Suppl 5):S1–S85.
-
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21:1414–1431.
-
Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol 2005; 139:498–503.
-
Saad MJ. Molecular mechanisms of insulin resistance. Braz J Med Biol Res 1994; 27:941–957.
-
Rocha EM, de MLMH, Carvalho CR, et al. Characterization of the insulin-signaling pathway in lacrimal and salivary glands of rats. Curr Eye Res 2000; 21:833–842.
-
Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 1992; 110:537–540.
-
Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001; 108:586–592.
-
Nepp J, Abela C, Polzer I, et al. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea 2000; 19:487–491.
-
Akinci A, Cetinkaya E, Aycan Z. Dry eye syndrome in diabetic children. Eur J Ophthalmol 2007; 17:873–878.
-
Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol 2000; 84:19–21.
-
Rahman A, Yahya K, Ahmed T, Sharif-Ul-Hasan K. Diagnostic value of tear films tests in type 2 diabetes. J Pak Med Assoc 2007; 57:577–581.
-
Hann LE, Kelleher RS, Sullivan DA. Influence of culture conditions on the androgen control of secretory component production by acinar cells from the rat lacrimal gland. Invest Ophthalmol Vis Sci 1991; 32:2610–2621.
-
Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci 1993; 34:2672–2679.
-
Cunha DA, Carneiro EM, Alves Mde C, et al. Insulin secretion by rat lacrimal glands: effects of systemic and local variables. Am J Physiol Endocrinol Metab 2005; 289:E768–E775.
-
Alves M, Calegari VC, Cunha DA, et al. Increased expression of advanced glycation end-products and their receptor, and activation of nuclear factor kappa-B in lacrimal glands of diabetic rats. Diabetologia 2005; 48:2675–2681.
-
Modulo CM, Jorge AG, Dias AC, et al. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocrine 2009; 36:161–168.
-
Partanen J, Niskanen L, Lehtinen J, et al. Natural history of peripheral neuropathy in patients with noninsulin-dependent diabetes mellitus. N Engl J Med 1995; 333:89–94.
-
Woost PG, Brightwell J, Eiferman RA, Schultz GS. Effect of growth factors with dexamethasone on healing of rabbit corneal stromal incisions. Exp Eye Res 1985; 40:47–60.
-
Saragas S, Arffa R, Rabin B, et al. Reversal of wound strength retardation by addition of insulin to corticosteroid therapy. Ann Ophthalmol 1985; 17:428–430.
-
Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol 2007; 125:1082–1088.
-
Walsh NP, Fortes MB, Raymond-Barker P, et al. Is whole-body hydration an important consideration in dry eye? Invest Ophthalmol Vis Sci 2012; 53:6622–6627.
** This study brings for the first time a proof for the hypothesis that dehydration may take part in the mechanisms of DES. They identified more DES symptoms in dehydrated patients.
-
Grus FH, Sabuncuo P, Dick HB, et al. Changes in the tear proteins of diabetic patients. BMC Ophthalmol 2002; 2:4.
-
Cade-Jorge F, Cade-Jorge I, Kusabara AA, Rocha EM. Perspectives in therapeutic innovation in ocular surface disorders and dry eye syndrome. Recent Pat Endocr Metab Immune Drug Discov 2009; 3:194–199.
-
Cole BE. Diabetic peripheral neuropathic pain: recognition and management. Pain Med 2007; 8 (Suppl 2):S27–S32.
-
Shternhall-Ron K, Quintana FJ, Perl S, et al. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun 2007; 28:134–142.
-
Hagberg CE, Mehlem A, Falkevall A, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 2012; 490:426–430.
* This is a preclinical study showing that systemic treatment with anti-VEGF antibody improves insulin sensitivity and metabolic control in rat models of diabetes mellitus.
-
Ye F, Shi B, Wu X, et al. Experience with lentivirus-mediated CD40 gene silencing in a mouse model of Graves’ disease. J Endocrinol 2011; 208:285–291.
-
Voutetakis A, Kok MR, Zheng C, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A 2004; 101:3053–3058.
-
Di Pasquale G, Dicembrini I, Raimondi L, et al. Sustained exendin-4 secretion through gene therapy targeting salivary glands in two different rodent models of obesity/type 2 diabetes. PLoS One 2012; 7:e40074.
** This is a preclinical study showing the feasibility of viral vector containing an agonist of Glucagon-like peptide to control glycemic levels in mice.
-
Voutetakis A, Bossis I, Kok MR, et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol 2005; 185:363–372.
-
Rocha EM, Cotrim AP, Zheng C, et al. Recovery of radiation-induced dry eye and corneal damage by pre treatment with adenoviral vector-mediated transfer of erythropoetin to the salivary glands in mice. Human Gene Ther 2013; 24:417–423.
* This recent publication shows for the first time in rodent models, that anabolic hormone therapy with erytropoethin using viral vector gene therapy can be useful to protect ocular surface and revert DES.
-
Rocha EM, Di Pasquale G, Riveros PP, et al. Transduction, tropism, and biodistribution of AAV vectors in the lacrimal gland. Invest Ophthalmol Vis Sci 2011; 52:9567–9572.
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 365–366).