More and more, PANOPTIX or Trifocal Intraocular lenses (IOL) are showing excellent results for distance, intermediate, and near vision. However, patients who have had LASIK are not FDA approved to have this wonderful IOL implant.

![]()



More surgeons are performing this procedure in post-LASIK patients with excellent results, but long term effects are still unknown.
I could not find a paper yet with a randomized evaluation of PANOPTIX versus Symfony in Post-LASIK patients, but this is a good review.
https://crstodayeurope.com/articles/2017-jun/multifocal-and-trifocal-lenses-in-post-lasik-eyes/
EDITORIAL SPOTLIGHT | JUNE 2017
Multifocal and Trifocal lenses in Post-LASIK Eyes
Considerations for implanting premium IOLs in patients with previous refractive surgery.
Cyres Keiki Mehta, MD; and Peter Mojzis, MD, PhD, FEBO

POSTOPERATIVE ASTIGMATISM: THE ENEMY OF MULTIFOCAL IOLS
The conundrum in post-LASIK cases is determining the appropriate IOL power.
With the advent of refractive surgery, particularly LASIK, in the 1990s, patients finally gained access to a safe and painless option to achieve spectacle independence. Many individuals in this early patient population have since developed presbyopia, and some have developed cataract due to aging, diabetes, trauma, or a multitude of other causes. However, they still maintain their desire to be spectacle-free.
The most frequent comment anterior segment surgeons hear when counseling this subgroup of cataract or presbyopic patients is, “I don’t want to wear glasses after cataract surgery; what are my options?” or, in the case of presbyopes, “I don’t want to wear reading glasses at all.”
Typically, we can exercise two options in these cases. In presbyopes, we can lift the LASIK flap and perform laser blended vision or another excimer laser strategy. Laser blended vision essentially creates monovision, wherein the dominant eye is corrected to -0.50 D and the nondominant eye to -1.25 to -1.50 D. The ablation also provides increased depth of focus by increasing spherical aberration. The difficulty with this approach is that some patients, having had excellent distance vision all these years, do not want one eye blurred for distance; they also want to read fine print, whether on their phones or devices or in books, as they did when they were younger.
In patients with a visually significant cataract, the only option is to implant a multifocal IOL. Today, I most often offer these patients a trifocal design—the AT LISA tri (Carl Zeiss Meditec; Figure 1) or the FineVision trifocal (PhysIOL; Figure 2)—or the extended range of vision Symfony IOL (Johnson & Johnson Vision). These lenses will be joined by the AcrySof IQ PanOptix (Alcon) once it is available in my country. In India, the AT LISA tri is available in a toric version, but the FineVision is not. In patients with more than 0.50 D cylinder, I always opt for a toric lens.
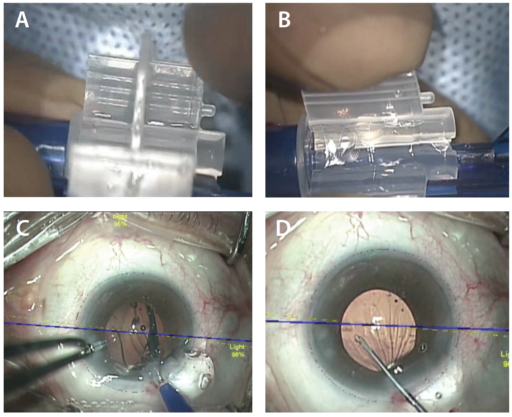
Figure 1. AT LISA tri: Preloaded (A) and in the injector (B). Injecting the AT LISA tri with toric overlay (C). Aligning the toric IOL with the markerless overlay system (D).

Figure 2. Injecting the FineVision trifocal IOL.
THE CONUNDRUM
The conundrum in these cases is which power of lens to implant so that the final result is as close to emmetropia as possible. All of the lenses mentioned here are aspheric. Studies show that spherical aberration increases significantly after myopic LASIK, degrading overall image quality. An aspheric IOL helps to compensate for the increased spherical aberration after myopic LASIK.1
In planning for cataract surgery in post-LASIK eyes, traditional IOL power calculation formulas no longer apply. Refractive outcomes after cataract surgery in eyes with previous myopic excimer laser surgery are unpredictable. The laser ablation for a myopic correction makes the anterior corneal surface flatter than that in an untreated eye. The keratometry (K) readings for this flatter cornea are thus lower than in a normal eye. The ratio between anterior and posterior corneal curvatures is also altered. Simultaneously, the asphericity of the anterior cornea is changed from prolate to oblate. In an oblate cornea, the K reading in the paracentral area is higher than at the center; it resembles a tabletop shape, so IOL power is underestimated by standard power calculation formulas. With third-generation theoretical formulas such as the SRK-T and Hoffer-Q, the underestimations in IOL power in these eyes can be significant.2
These formulas calculate the effective lens position (ELP) under the assumption that the cornea has a spherical shape. The ELP is estimated as a function of the presumed anterior chamber depth, which is geometrically determined by the K reading. In eyes with previous myopic LASIK, the ELP is underestimated because of the flattened cornea, whereas the actual anterior chamber depth does not change. As a result, the IOL power is underestimated, and the postoperative refractive outcome is a hyperopic error.3 This hyperopic surprise, in which the patient presents after cataract surgery with an error between 1.50 and 3.00 D, or even more, was common in the past after IOL calculation with the standard SRK-T method, but it is now rare.4,5
PREOPERATIVE ASSESSMENTS
Before cataract surgery or refractive lens exchange (RLE) for presbyopia, post-LASIK eyes should undergo the following workup.
Corneal topography. Accurate corneal topography measurements allow the surgeon to place the phaco incision on the steep axis and, thus, minimize postoperative astigmatism—the enemy of multifocal IOLs. Corneal topography can also reasonably estimate central corneal power, and this value can be used in IOL power calculation formulas. Simulated K is of less relevance. The Atlas Corneal Topography System (Carl Zeiss Meditec) has been noted in the literature to be a useful tool for measuring central corneal power.6
Ray-tracing software such as that of the iTrace (Tracey Technologies) can provide additional information about corneal aberrations and can distinguish between those on the corneal surface and those caused by cataract formation.
Accurate axial length measurement. Immersion A-scan biometry (Axis; Quantel Medical) or optical biometry with the IOLMaster 700 (Carl Zeiss Meditec) or the Lenstar (Haag-Streit) should be used to calculate axial length. This measurement is important because errors in axial length lead directly to errors in IOL power.
Dilated fundus examination. If there are problems at the macula, such as an impending macular hole or dry age-related macular degeneration, multifocal IOL implantation should be avoided. In addition to dilated fundus exam, OCT should be used to evaluate the macula in these patients.
Anterior segment OCT. Anterior segment OCT can reveal the exact corneal thickness, LASIK flap thickness, and quality of the stromal bed. This technology is also helpful if the lens power calculation goes awry and we have to lift the flap and use the excimer laser for a postoperative adjustment.
IOL power calculation. Typically, I prefer to use the Haigis-L formula (myopic or hyperopic), which is included in the software of the IOLMaster 700. The IOLMaster 700 has many more keratometric points than the IOLMaster 500, and it uses swept-source OCT for axial length measurement as compared with laser interferometry in the IOLMaster 500.7
The Barrett True-K formula has been shown to be either equal to or better than alternative methods available on the ASCRS online calculator for predicting IOL power in eyes with previous myopic LASIK or PRK.
The Barrett True-K formula is based on the Barrett Universal II formula. It calculates a modified K value for patients who have had myopic or hyperopic LASIK or PRK or RK, and it requires the measured K reading and the pre- and postlaser refraction values. The Barrett True K formula can predict the required power of the IOL when no refractive history is available (no-history formula). It can also be used with or without considering the surgically induced change in refraction (change in manifest refraction) because it uses an internal regression formula to calculate an estimated change in manifest refraction when those data are not entered.
For all postrefractive surgery cases, I use the ASCRS online calculator. This online calculator has recently been revised to include new, innovative formulas such as the Barrett True-K and the Potvin-Hill formula, which uses input from a Scheimpflug device (Pentacam; Oculus) and OCT.
PRESBYOPIC RLE
For presbyopic RLE (Figure 3), patients must be 48 years or older; below this, the degree of presbyopia does not justify the procedure. Patients aged 43 or 44 years do not have a sufficient degree of presbyopia and, therefore, after the procedure often feel that their preoperative vision with their residual accommodation was better. Additionally, the rate of retinal detachment after Nd:YAG capsulotomy is unacceptably high in this young group of presbyopes (personal communication from Sheraz M. Daya, MD, FACP, FACS, FRCS(Ed), FRCOphth). Patients undergoing presbyopic RLE must consent that some amount of glare and halos is tolerable. It is advisable to avoid performing the procedure in low myopes; for example, a patient with -1.00 D will be happier without surgery.

Figure 3. Presbyopic RLE.
Surgeons should exercise caution when performing presbyopic RLE. Remember that you are operating on patients who have 20/20 CDVA and N5 CNVA. Any shortcoming in surgery will be immediately picked up by the patient. The femtosecond laser is an efficient tool in this setting, as it can be used to perform limbal relaxing incisions if a toric IOL is not available; further, the capsulorrhexis position and size will be perfect in every case.
CONCLUSION
The happiest patients after lens surgery for presbyopia are:
• Hyperopes, as spectacle independence for far and near can be achieved;
• Patients with cataracts and poor vision preoperatively, as the improvement in vision is gratifying; and
• Patients who refuse to wear spectacles for cosmetic reasons and are presbyopic before surgery.
• Patients with cataracts and poor vision preoperatively, as the improvement in vision is gratifying; and
• Patients who refuse to wear spectacles for cosmetic reasons and are presbyopic before surgery.
Refractive surprises after monovision LASIK can occur because the surgical corneal power is underestimated or because the IOL position was not accurately predicted. Before cataract surgery, post-LASIK eyes should undergo corneal topography, accurate axial length measurement, dilated fundus examination, and anterior segment OCT. If IOL power calculation goes grossly wrong, the option to exchange the lens always exists.

THE IDEAL SURGICAL SOLUTION
These lenses can provide excellent spectacle independence and improve intermediate visual acuity without sacrificing distance and near vision in the post-LASIK eye.
By Peter Mojzis, MD, PhD, FEBO
Over the past decade, the number of patients who present for cataract surgery with a history of LASIK has increased dramatically. These patients are familiar with refractive procedures, are of presbyopic age, and seek options for improving their visual acuity across all distances. In my experience, trifocal IOLs are an ideal surgical solution in these challenging cases.
EVALUATING PREVIOUS LASIK
Many patients with a history of refractive surgery present without any preoperative data. Often, they do not even know whether they underwent hyperopic or myopic LASIK. Thus, the first step in planning cataract/IOL surgery is identifying the type of LASIK correction they received. This is done by looking for evidence of central steepening (indicating hyperopic LASIK) or central flattening (indicating myopic LASIK).
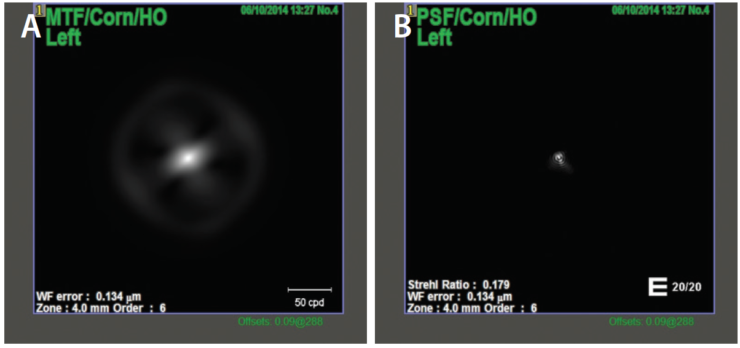
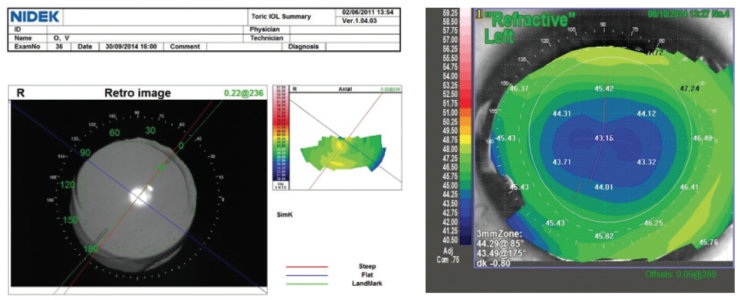
I use the OPD-Scan III (Nidek) to evaluate corneal topography, LASIK flap centration, higher-order aberrations (HOAs)—mainly coma and spherical aberration—and angle kappa. Additionally, I find it useful to assess the preoperative optical quality of the whole optical system, the lens, and especially the cornea by looking at point spread function (PSF) and modulation transfer function (MTF). PSF measures the intensity of light in the image of a point source, and MTF evaluates the loss of contrast when light passes through an optical system (Figure 4).

Figure 4. MTF (A) and PSF (B) corneal values in a patient with previous myopic LASIK scheduled for trifocal IOL implantation, measured in a 4-mm pupil. The Strehl ratio for HOAs was high at 0.179.
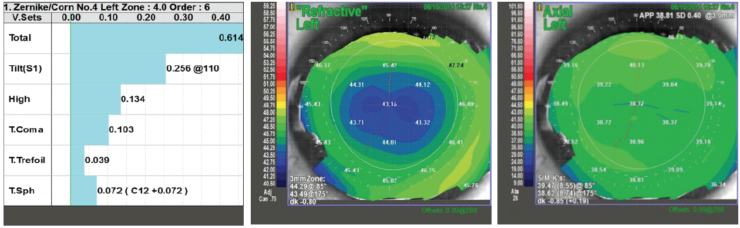
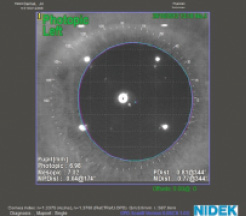
Decentered LASIK flaps, postoperative ectasia, and high degrees of HOAs can decrease optical performance after lens surgery. Corneal refractive surgery modifies the shape of the cornea and can change or induce HOAs. Use of a multifocal IOL in an aberrated cornea could worsen optical quality and cause symptoms of double vision, blurred vision, halos, or glare. The magnitude of HOAs is determined by, among other factors, ablation profile, flap quality, and pupil size. The size and severity of corneal aberrations should be evaluated using wavefront aberrometry before multifocal IOL implantation is planned (Figure 5).
An abnormal corneal shape can also induce regular or irregular corneal astigmatism. To minimize postoperative refractive error in an eye with a high degree of corneal astigmatism, I implant a trifocal IOL with a toric component (Figure 6).

Figure 5. Low induced HOAs, coma, and spherical aberration are demonstrated in this eye after myopic LASIK, measured in a 4-mm pupil. Corneal topography shows a well-centered optical zone.

Figure 6. A trifocal toric IOL centered in the capsular bag in a patient with previous hyperopic LASIK.
ANGLE KAPPA
Angle kappa is the angle between the visual and pupillary axes. A high angle kappa can play a significant role in postoperative lens decentration, resulting in increased glare and halos.
Hyperopes especially tend to have large angle kappa values. I conducted a study in which angle kappa was measured in 60 eyes (30 patients). In 42 hyperopic eyes, mean angle kappa was 0.43 ± 0.18; in six emmetropic eyes, mean angle kappa was 0.34 ± 0.12; and in 10 myopic eyes, mean angle kappa was 0.2 ± 0.1.
In patients with a small angle kappa after myopic LASIK, I recommend centering a trifocal lens on the corneal vertex; in patients with large angle kappa after hyperopic LASIK, the lens should be centered halfway between the pupillary and corneal reflexes. In some cases, at the end of surgery, intracameral carbachol intraocular solution may be used to produce miosis. Induced miosis is a simple and effective method for centering a multifocal IOL in patients with small angle kappa, wherein the visual axis is close to the pupillary axis. The best point for perfect multifocal IOL centration in patients with a high degree of angle kappa is the first Purjkinje image, which is close to visual axis. The AT LISA tri has a large central optical zone of 1.04 mm, which is an excellent solution for patients with large angle kappa (Figure 7).

Figure 7. An eye with a large angle kappa. The white point indicates the corneal vertex and the blue point the pupil center.
IOL CALCULATION
IOL calculation in patients with previous refractive surgery is less predictable than in virgin eyes. Alterations of the anterior corneal curvature induced by flap creation and excimer laser ablation may lead to miscalculations. The main error in IOL calculations in post-LASIK eyes arises from the use of a formula with a normal anterior:posterior corneal curvature ratio. After myopic LASIK, this calculation overestimates corneal curvature and leads to implantation of an IOL with too low dioptric power and a resultant hyperopic shift. Residual hyperopia worsens visual acuity at all distances. In such an event, previously myopic patients are highly dissatisfied.
Many methods have been presented to help calculate IOL power more precisely. I recommend using the Wang-Koch-Maloney method. This method is simple: The formula is (CP x 1.114) – 6.1 = estimated corneal power, where CP is the value of central corneal power obtained upon placing the cursor in the center of the axial topography map.
Hyperopic LASIK modifies the peripheral cornea, increasing the anterior:posterior corneal radius; thus, calculations after hyperopic LASIK are easier than those after myopic LASIK, in which the central corneal curvature is modified. In my practice, in patients without access to their previous medical reports, I exclusively use the hyperopic Haigis-L formula.
Regarding surgical approach, the placement of incisions should avoid the LASIK flap. Corneal incisons should be placed outside the flap to minimize damage by means of phaco tip and/or cannulas or accidental instillation of balanced saline solution or OVD. In the event postoperative fine-tuning is needed, there is no problem lifting the damaged LASIK flap.
CONCLUSION
Cataract surgery in patients with previous myopic or hyperopic LASIK is challenging for a number of reasons. Precise IOL calculation is essential, and assessments of HOAs, corneal astigmatism, angle kappa, and preoperative optical quality are all key factors for obtaining excellent postoperative results.
In my experience, the AT LISA tri and AT LISA tri toric and the Alsafit trifocal and Alsafit trifocal toric (both from Alsanza) can provide excellent spectacle independence and improve intermediate visual acuity without sacrificing distance and near vision in post-LASIK patients. These lenses are stable, they center well in the capsular bag, and they provide normal contrast sensitivity—all notable advantages in these complex surgical scenarios.
EDITORIAL SPOTLIGHT | JUNE 2017
Monovision: Underestimate at Your Peril
Monovision remains the best competition to more sophisticated options such as multifocal and trifocal IOLs.
Arthur B. Cummings, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)

Monovision. One vision. One eye for distance vision, one eye for near. Sounds strange at first. However, if the patient is deemed a good candidate following a comprehensive evaluation, if the targets have been carefully chosen, and if the compromise is understood, then I contend that multifocal and trifocal IOLs will have to be very good indeed to compete with monovision.
THE COMPROMISE
What is the aforementioned compromise? Once a patient reaches age 45 and older, at present there is no solution to provide him or her with the vision of a 20-year-old emmetrope. When a presbyope comes for a consultation, therefore, our conversation often starts with, “We are going to try to find the compromise that suits you best.” Put another way, “We are going to try to provide you with the most functional vision for your needs.”
AT A GLANCE
• Using appropriate language and analogies can help patients understand the limitations of monovision.
• A careful history allows the surgeon to determine where the best target should be, given the patient’s needs.
• Any aspect of monovision that is not perfectly tolerated can be remedied with a pair of spectacles for a specific task, although the percentage of patients requiring glasses is likely to be low.
Compromise No. 1. The first compromise might be perfect distance vision, targeting emmetropia and hopefully better than 6/6 UDVA in both eyes with a high-fidelity outcome. Where is the compromise here? It is the fact that, if the patient is presbyopic or pseudophakic with a monofocal IOL in situ, he or she cannot read without reading glasses.
Compromise No. 2. The second compromise may be a premium IOL with multifocal or trifocal optics. Here, the compromise is not the difference between the two eyes, but rather the way in which light behaves in each eye. Trifocal IOLs and extended depth of focus IOLs have both provided excellent compromises in first-time procedures, but they remain a compromise, with all light entering the eye being separated into different focal points. And the compromise is maybe a little greater in an eye with previous corneal refractive surgery, depending on the quality of the corneal surgery and the resultant asphericity.
Compromise No. 3. The third compromise to contemplate is correcting both eyes for perfect near vision (just for illustration, as few would opt for this choice). Now the patient’s reading vision is perfect, but the compromise lies in the fact that he or she requires glasses for distance vision.
Here is where monovision becomes more understandable to patients: “So, can I leave one eye slightly short-sighted, providing me better near vision, while the other eye provides excellent distance vision?” The answer is, “Yes, you can, if you find the compromise satisfactory and better than the other compromises I described.”
Another comment that makes monovision eminently understandable is, “Today, many of us are required to see well at three distances: near, intermediate, and far. The problem is that we have only two eyes. To see perfectly at all three distances would require three eyes.” Patients immediately grasp the limitations of monovision when they hear this explanation.
ESTABLISHING CANDIDACY AND TARGETS
How does one establish that a patient is a good candidate for monovision, and how do we determine targets? I normally demonstrate full correction through the auto-phoropter and ask the patient to rate this as 100% and consider this his or her reference point for the rest of the evaluation. I then show the patient one eye fully corrected for distance vision and the other under-corrected by -1.50 D for starters. I demonstrate this both ways—that is, with the left eye for distance and then with the right eye for distance. Typically, the patient will prefer one over the other. If he or she does not have a preference, then we typically advise patients who drive a lot to correct the right eye for distance (if they drive on the left side of the road; it would be the opposite for those living in continental Europe or the United States).
Once you have established the sensory dominance as described above (the near-far combination that feels more comfortable), then the refractive target for the reading eye is determined. Once more, both eyes are fully corrected, and the distance stereo chart is projected. At this point, the patient can normally see which bars stand out more prominently on the stereo chart. Once stereo vision is established, then the nondominant eye is defocused by adding plus until the distance stereo vision is compromised. When this happens, we have gone too far, and then some of the plus is removed until the stereo vision returns.
Many patients still have full distance stereo vision with full correction in one eye and -1.25 D under-correction in the reading eye. Some can go to -1.50 D or even higher and still have decent distance stereo vision. This evaluation has just determined the limit of the defocus for the reading eye (see video below).
A careful history then allows the surgeon to determine where the best target should be, given the patient’s needs. For example, someone who uses a computer all day will likely prefer plano/-1.00 D to plano/-1.75 D, even if he or she could tolerate the greater anisometropia and maintained stereopsis for distance.
A visual lifestyle monitor (Vision Lifestyle Monitor; Vivior) can provide clinicians with objective data of exact working distances and time spent at these distances in an efficient manner. This personal defocus curve can then be treated with a level of monovision that suits the patient’s visual surroundings closer than any conversation or questionnaire can. Consider that a 6’6” man calls reading vision or reading a book the same as a 5’4” person, yet their working distances are completely different.
Any aspect of monovision that is not perfectly tolerated can be remedied with a pair of spectacles for a specific task, such as driving at night. In my practice, 7% of patients with monovision wear glasses when driving at night, but 93% are entirely free from any glasses whatsoever. Before resorting to glasses, one can also offer brain training exercises that work well for most patients who are struggling with some aspect of monovision (see Video below).
CONCLUSION
Even dismissing the cost benefits of monovision, from a performance perspective it remains the biggest competition to more sophisticated options such as multifocal and trifocal IOLs or corneal inlays. It is my belief that, if monovision is assessed and managed as I have just outlined, there is the potential to further increase its acceptance.
EDITORIAL SPOTLIGHT | JUNE 2017
Use of the Light Adjustable Lens after Corneal Refractive Surgery
Postoperative adjustability is a major advantage in these challenging eyes.
Fritz Hengerer, MD, PhD

Performing cataract surgery in patients who have undergone corneal refractive surgery can be challenging, as preoperative biometry is often unreliable due to the influence of the earlier surgery. This can lead to selection of the wrong IOL in these patients who are accustomed to glasses-free vision and tend to have high visual expectations. RxSight’s Light Adjustable Lens (RxLAL; Figure 1) may be the most predictable way to provide optimal visual outcomes for these patients, as the lens can be precisely optimized after its implantation if the desired refractive result is not achieved.
AT A GLANCE
• Performing cataract surgery in patients who have undergone corneal refractive surgery can be challenging, as preoperative biometry is often unreliable due to the influence of the earlier surgery.
• Instead of relying on keratometry values, prediction of effective IOL position, and various IOL formulas for eyes with altered corneas, it may be better to adjust the refraction postoperatively.
• With the RxLAL, up to three office-based adjustments, each taking approximately 90 seconds, can be made to fine-tune refractive outcomes.
THE LIGHT ADJUSTABLE LENS
The RxLAL is adjusted postoperatively with an office-based Light Delivery Device (LDD) that resembles a slit-lamp Nd:YAG laser. The LDD delivers a predetermined pattern and amount of UV-A light (365 nm wavelength) to the photosensitive silicone lens, inducing precise modifications in lens curvature based on principles of photochemistry and diffusion.


Figure 1. Illustrations show the three-piece design of the RxLAL (Above) and its position after implantation (Below).
The photosensitive lens incorporates proprietary silicone photoreactive additives called macromers, which are distributed throughout the lens. When light is directed to a specific area of the lens, macromers in the path of the light attach to the ends of other macromers, forming polymers. The remaining unreacted macromers physically diffuse into the exposed area, causing a highly predictable change in the curvature of the lens. As long as there are unpolymerized macromers, further adjustments to refine the refractive power can be made by applying light to different areas of the lens.
Utilizing a sub–3-mm clear corneal incision, the RxLAL is implanted like any standard foldable monofocal IOL. Its three-piece design features a 6-mm optic diameter and 13-mm overall length. After a period of postoperative refractive stabilization (approximately 2 weeks after surgery), the lens is adjusted noninvasively. Postoperative adjustments can range from -2.00 to 2.00 D sphere and from 0.50 to 3.00 D cylinder in any combination.
Up to three office-based adjustments, each taking approximately 90 seconds, can be made to fine-tune refractive outcomes. Once the patient’s vision is optimal, the IOL is permanently locked in at its current refractive state, preventing further power changes. The RxLAL is sensitive to UV-A light until the adjustment process is completed, so, until final lock-in, patients must wear UV-protective glasses to avoid incidental exposure from sunlight.
A prospective, nonrandomized clinical trial including 122 previously unoperated eyes of 91 patients with significant cataract (virgin eyes) was conducted at the Center for Vision Science, Ruhr University Eye Clinic, in Bochum, Germany. Postoperatively, 98% of eyes were within 0.50 D of target manifest refraction spherical equivalent (MRSE), 100% had UCVA of 20/25 or better, and 88% had UCVA of 20/20 or better.1
John F. Doane, MD, a clinical investigator in the pivotal study for US FDA approval of the lens, shared similar study outcomes at the 2017 American-European Congress of Ophthalmic Surgery (AECOS) Winter Symposium in Aspen, Colorado. In that study, more than 90% of 391 RxLAL eyes were within 0.50 D of target MRSE, 92% had a UCVA of 20/25 or better, and more than 70% had a UCVA of 20/20 or better. In both studies, nearly 30% of eyes had a UCVA of 20/16 or better.2
By comparison, in a recently published meta-analysis of toric IOLs,3 only 65% of eyes achieved 20/25 or better postoperative UCVA.
AFTER CORNEAL REFRACTIVE SURGERY
Postoperative adjustability clearly reduces the degree of error from imprecision in biometry, power calculation, and postimplantation movement of the IOL. However, this problem becomes even more challenging in eyes that have undergone previous LASIK, PRK, or another corneal refractive surgical procedure, due to additional abnormalities from irregular corneal surfaces.
LASIK is the most common refractive surgical procedure in ophthalmology today, and many who have undergone the procedure are now reaching cataract surgery age. The RxLAL may, therefore, present a superior approach for achieving desired outcomes of cataract surgery in these post-LASIK eyes. Instead of relying on keratometry values, prediction of effective IOL position, and various IOL formulas for eyes with altered corneas, it may be better to simply adjust the refraction postoperatively. This would reduce the burden of having to achieve emmetropia immediately after surgery in these complicated cases.
Results with the RxLAL in postrefractive surgery eyes have been published, most notably by Brierley.4 In this retrospective study of 34 postrefractive surgery eyes in 21 patients, the MRSE relative to target refraction after final lock-in with the RxLAL was within ±0.25 D in 74% of eyes, ±0.50 D in 97% of eyes, and ±1.00 D in 100% of eyes. These results were 60% more predictable than the best refractive outcomes achieved in previous studies with monofocal IOLs in postrefractive surgery eyes.4
When I practiced at Ruhr University Eye Clinic, I participated in a multicenter, international, collaborative analysis to better understand the clinical results in postrefractive surgery eyes implanted with the RxLAL. This retrospective analysis, performed by four surgeons in three countries, included 37 eyes with a history of at least one corneal refractive procedure (LASIK, PRK, RK, or CK) prior to RxLAL implantation. Twenty eyes were targeted for distance (emmetropia) and 17 for near vision (adjustable monovision).
Analysis of the distance-corrected eyes (n=20) showed that 70% of eyes achieved a postadjustment UDVA of 20/25 or better, with an overall improvement from preadjustment UDVA to postadjustment UDVA of more than 2 lines. Only 40% of eyes were within ±0.50 D MRSE before adjustment, but this improved to 95% (19 of 20) after the lens was adjusted and locked in. The postadjustment MRSE showed a marked improvement over published results with the modified Masket method, in which 67% of previous LASIK and PRK patients were reported within ±0.50 D of target MRSE.5
Seventeen eyes in this analysis were targeted for near vision (adjustable monovision), with a mean target refractive goal of -1.50 D. After adjustment and lock-in, the average MRSE was -1.50 ±0.55 D, and average UNVA was 20/25 (J2), demonstrating that near vision eyes implanted with the RxLAL can be adjusted to achieve the proper amount of desired myopia.
CONCLUSION
In my experience, the RxLAL is an effective and promising option for avoiding the inaccurate biometry readings and managing refractive surprises that are often seen after IOL implantation in postrefractive surgery eyes. Due to its postoperative adjustability, the RxLAL offers a level of refractive precision superior to that of nonadjustable IOLs. Additionally, this group of patients that has previoulsy undergone refractive surgery understands the value of excellent uncorrected vision and is generally willing to pay for a superior outcome.
Other benefits of the RxLAL include improved binocular accuracy with monovision treatments. Although monovision is a common strategy used to address presbyopia, the surgeon’s ability to achieve the desired offset and patient tolerance can be highly unpredictable. With the RxLAL, not only is the surgeon able to offer a more accurate visual outcome, but also the patient can preview the outcome, the surgeon can then adjust the monovision offset as desired.
1. Hengerer F, Dick B, Conrad-Hengerer I. Clinical evaluation of ultraviolet light adjustable intraocular lens implanted after cataract removal. Ophthalmology. 2011;118:2382-2388.
2. Doane J. Prediction to prescription—the future of cataract surgery. Paper presented at: the American-European Congress of Ophthalmic Surgery. February 26-March 1, 2017; Aspen, Colorado.
3. Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery – a systematic review and meta-analysis. Ophthalmology. 2016;123:275-286.
4. Brierley L. Refractive results after implantation of a light-adjustable intraocular lens in postrefractive surgery cataract patients. Ophthalmology. 2013;120:1968-1972.
5. Wang L, Hill W, Koch D. Evaluation of intraocular lens power prediction methods using the American Society of Cataract and Refractive Surgeons post-keratorefractive intraocular lens power calculator. J Cataract Refract Surg. 2010;36:1466-1473.




