PRP Injections into LACRIMAL GLAND Protocol for Dry Eye Disease
Lacrimal Gland Injections of Platelet Rich Plasma (LaGPRiP)
The goal of these treatments is to help relieve eye discomfort and pain due to damaged tissue from other factors.This protocol is experimental but has been published as an effective remedy for patients with aqueous deficiency.
PRP into the Meibomian gland is also experimental but we have had successful injections in multiple patients and hope to publish our results: see https://drcremers.com/2017/09/injection-of-platelet-rich-plasma-into.html?showComment=1511303115123
Many patients have contacted me from around the world search for a cure for their chronic eye pain. Many have seen various surgeons and have tried multiple options without relief.
I hope Lacrimal Gland Injection of Platelet Rich Plasma will give long term relief to these patients.
PROTOCOL:
Here is a brief example of our current protocol.
Please contact Jen Ramirez at jramirez@voeyedr.com if you would like more information or to schedule a consult.
- Consent form signed
- Oxford Scheme Grading Sheet: see below
- PRP made according to sterile, published technique
- Schirmers Test I
- Lissamine green test
- Photos taken for before and after treatment
- Ideally we need SJO test done on all these patients. Ask for any old blood work prior to visit. If patient is positive for Sjogren’s syndrome, this procedure is more likely to work according to published data.
- Right before Lacrimal Gland injection of PRP:
- proparacaine 0.5%: 1 drop in inferior fornix
- 5% Betadine: 1 drop in inferior fornix
- Antibiotic drop: 1 drop in inferior fornix
- 30-gauge tuberculin syringe with 1ml of PRP
- Demarres retractor: small or medium size: have sterile one in sterile package ready in room.
- Prescriptions Rx needed after each injection:
- NeoPoly Dexa drops qid and
- NeoPoly Dexa ointment qhs in treated eye x 4 days after each injection
- Samples of Non Preserved Artificial Tears
- Give rest of PRP to use bid-qid till next visit
- Post Op Instruction Sheet given
Prior to procedure, all patients need the following:
Surgical to schedule Day 1, Week 4, 8, 12: so need 4 appointments booked.
- Sign consent form; Techs & Scribes be sure Consent form is signed
- Meibography of all 4 eyelids within 3 weeks of procedure: ideally the day of the procedure.
- Obtain blood to make PRP:
- Schirmer Test 1 (without anesthesia) by Dr. Cremers
- Evaluation of cornea after Lissamine green 1% solution placement.
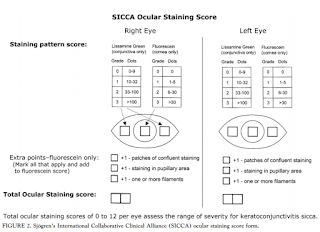
- Apply to inferior fornix; Grade according to Oxford Scheme: measure nasal, corneal, temporal areas: Maximum sum=12: see grading below: print out of grading for each patient for Dr. Cremers to fill out; scan when done
- 0-9 dots seen: Grade0
- 10-32 dots: Grade 1
- 33-100 dots: Grade 2
- >100 dots: Grade 3
- Filaments seen: add point
- Lacrimal Gland Injection PRP (LaGPRiP)
- Patient to return for same protocol at Week 4, 8, 12
References:
- Cornea. 2014 Jan;33(1):18-21. doi: 10.1097/ICO.0000000000000016.
Restoration of human lacrimal function following platelet-rich plasma injection.
Avila MY1.
PURPOSE:The aim was to evaluate the effect of autologous platelet-rich plasma on lacrimal function in patients with severe dry eye.METHODS:A prospective interventional case series design was adopted. Four patients with severe lacrimal dysfunction and severe dry eye were treated. Platelet rich-activated plasma (1 mL) was injected adjacent to the lacrimal gland on day 0 and at 4, 8, and 12 weeks. The objective parameters included a Schirmer test, ocular surface staining, and tear break-up time (TBUT). The patients were followed up for 12 weeks after the first injection.RESULTS:All cases showed a significant improvement in lacrimal volume (from 3.3 ± 0.8 mm to 11.1 ± 2.3 mm). In all the patients, an increase in tear break-up time values and a decrease in ocular staining (basal 8.0 ± 0.61-2.8 ± 0.5) with subjective improvement occurred. None of the patients presented any adverse effect, and none reported pain or discomfort. Additionally, no complications were observed.CONCLUSIONS:Injected platelet-enriched plasma was found to be safe and effective in increasing lacrimal production and in improving ocular staining secondary to severe dry eye. This approach could be an alternative for the management of these patients, although additional studies are required to perfect the technique.
2.
Am J Ophthalmol. 2010 Mar;149(3):405-15. doi: 10.1016/j.ajo.2009.09.013. Epub 2009 Dec 29.
A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry.
Whitcher JP1, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, Hamann S, Larkin G, McNamara NA, Greenspan JS, Daniels TE; Sjögren’s International Collaborative Clinical Alliance Research Groups.
Author information
Abstract
PURPOSE:
To describe, apply, and test a new ocular grading system for assessing keratoconjunctivitis sicca (KCS) using lissamine green and fluorescein.
DESIGN:
Prospective, observational, multicenter cohort study.
METHODS:
The National Institutes of Health-funded Sjögren’s Syndrome International Registry (called Sjögren’s International Collaborative Clinical Alliance [SICCA]) is developing standardized classification criteria for Sjögren syndrome (SS) and is creating a biospecimen bank for future research. Eight SICCA ophthalmologists developed a new quantitative ocular grading system (SICCA ocular staining score [OSS]), and we analyzed OSS distribution among the SICCA cohort and its association with other phenotypic characteristics of SS. The SICCAcohort includes participants ranging from possibly early SS to advanced disease. Procedures include sequenced unanesthetized Schirmer test, tear break-up time, ocular surface staining, and external eye examination at the slit lamp. Using statistical analyses and proportional Venn diagrams, we examined interrelationships between abnormal OSS (>or=3) and other characteristics of SS (labial salivary gland [LSG] biopsy with focal lymphocytic sialadenitis and focus score >1 positive anti-SS A antibodies, anti-SS B antibodies, or both).
RESULTS:
Among 1208 participants, we found strong associations between abnormal OSS, positive serologic results, and positive LSG focus scores (P < .0001). Analysis of the overlapping relationships of these 3 measures defined a large group of participants who had KCS without other components of SS, representing a clinical entity distinct from the KCS associated with SS.
CONCLUSIONS:
This new method for assessing KCS will become the means for diagnosing the ocular component of SS in future classification criteria. We find 2 forms of KCS whose causes may differ.
—
Sandra Lora Cremers, MD, FACS
Johns Hopkins University Medicine, Suburban Hospital

Office: 301-896-0890
*One Central Plaza. 11300 Rockville Pike, Suite 1202. Rockville, MD 20852
*Van Ness Center. 4301 Connecticut Ave., NW, Suite 125. Washington, DC 20008
www.voeyedr.com
eyedoc2020.blogspot.com
Attention: This communication may contain protected health information (PHI) that is legally protected from inappropriate disclosure by the Privacy Standards of the Health Insurance Portability Act (HIPAA) and relevant Maryland Laws. If you are not the intended recipient, please note that any dissemination, distribution or copying of this communication is strictly prohibited. If you have received this message in error, you should notify the sender immediately by telephone or by return e-mail and delete this message from your computer.
Johns Hopkins University Medicine, Suburban Hospital
Office: 301-896-0890
*One Central Plaza. 11300 Rockville Pike, Suite 1202. Rockville, MD 20852
*Van Ness Center. 4301 Connecticut Ave., NW, Suite 125. Washington, DC 20008
www.voeyedr.com
eyedoc2020.blogspot.com
Attention: This communication may contain protected health information (PHI) that is legally protected from inappropriate disclosure by the Privacy Standards of the Health Insurance Portability Act (HIPAA) and relevant Maryland Laws. If you are not the intended recipient, please note that any dissemination, distribution or copying of this communication is strictly prohibited. If you have received this message in error, you should notify the sender immediately by telephone or by return e-mail and delete this message from your computer.