Migraines and it’s various classifications can be confusing.
Below is a rough summary of key types, questions to ask, and what to worry about.
Key Types:
When one hears the term ‘migraine,’ one often think of a severe headache. Migraine is considered, though, a neurological disease and that there are a number of different subtypes of migraine.
Migraine with Aura (Complicated Migraine)
About a quarter of people who experience migraine also experience aura, a series of sensory and visual changes that can range from seeing black dots and zig zags to tingling numbness on one side of the body, or an inability to speak clearly. Aura sets in shortly before or during a migraine and can last anywhere from 10 to 30 minutes. Aura is the second of migraine’s four stages, and anyone who experiences it will confirm it is an unmistakable warning sign that the severe head pain is on its way.
Migraine without Aura (Common Migraine)
Diagnosing migraine without aura can be difficult because the symptoms are similar to several other types of migraine. Pulsing or throbbing pain on one side of the head, photophobia, phonophobia, pain that is made worse by physical activity, and nausea and vomiting are all classic symptoms of Migraine without Aura. The key differentiator is that Common Migraine lacks the warning phases (prodrome and aura) that other types of migraine have.
Migraine Without Head Pain
Also called a Silent or Acephalgic Migraine, this type of migraine can be very alarming as you experience dizzying aura and other visual disturbances, nausea, and other phases of migraine, but no head pain. It can be triggered by any of a person’s regular triggers, and those who get them are likely to experience other types of migraine, too. The International Headache Society classifies this type as typical aura without headache.
Ocular (aka Retinal) Migraine: usually lasts <1hr but temporary blindness can last months.
When a headache causes you to temporarily lose vision in one eye, it is a Retinal Migraine. Patients will see colors, flashing lights. It is most common in women during their childbearing years, the blindness can last anywhere from a minute to months, but is usually fully reversible. This is a specific type of aura that accompanies a migraine, and it’s a condition we know very little about. What we do know is that Retinal Migraine may be a sign of a more serious issue, and those who experience it should make a point to see a specialist.
Hemiplegic Migraine
If you have ever had a migraine that felt more like a stroke, it was probably a Hemiplegic Migraine. People who experience this type of migraine develop weakness on one side of the body, often with visual aura symptoms and a “pins and needles” sensation, or loss of sensation, on one side of the body. It can last for as little as a few hours to several days. Similar to typical aura without headache, Hemiplegic Migraine doesn’t always include severe head pain.
Ophthalmoplegic Migraine
Chronic Migraine
If you have a headache more than 15 days a month, you’re probably suffering from chronic migraine. Many of the days often feel like typical migraine, but there may be considerable variability in the severity of the symptoms and head pain on any given day. Some days patients may mistake the pain for a “tension-headache” or “sinus headache” if the pain is less severe. Many patients with chronic migraine also use acute headache pain medications on more than 10-15 days per month, and this can actually lead to even more frequent headache.
.
Cluster Headaches
This is one of the most severe types of pain that a human can experience. With cluster headaches, you’ll feel an almost burning pain around and above your eyes, at your temples, and even moving toward the back of your head. You’ll often also get red or swollen eyes or a runny nose, among other symptoms. Because they occur in such a large area and provoke other symptoms, cluster headaches can be the most irritating headache, and are sometimes referred to as “suicide headaches.”
Cervicogenic headache
When the pain in your head is actually caused by pain in your neck, you probably have a cervicogenic headache. The pain usually comes from the neck or from a lesion on the spine, which is often confused with pain in the back of your head. It’s common for this type of headache to require physical therapy in addition to medication or other treatment.
Ice Pick Headaches
Ice pick headaches are pretty self-explanatory. They feel like you’re getting stabbed in the head with an ice pick. They often come on suddenly, delivering an intense, sharp pain. They’re short–usually only lasting 5-30 seconds–but incredibly painful. These headaches occur on the orbit, temple, and parietal area of your head. That’s where your trigeminal nerve is, which is the nerve in your face that’s responsible for biting and chewing, as well as face sensation. The nerve is on the side of your head just past your eye and above your ear. If you get sharp pains in this area, chances are you’re getting ice pick headaches
Diagnostic Recommendations: What Tests Should I order and When for Patients with Headaches or Migraines?
0. Get full history: ask about TMJ (think GCA/Temporal arteritis in those over 50); impotence/ED (think vascular association: see **); ask about depression, fatigue, weight loss, Polymyalgia Rheumatica (think GCA), as if gluten free (think diet triggers)
1. Full eye exam: recommend be done in all patients with Headaches/Migraines. Look at BCVA, check for APD, check retinal vessels carefully for spasm, narrowing, AION, Hollenhorst plaques; check Temporal Artery pulses bilaterally in patients over 50yo.
2. Pentacam: recommend in all phakic (ie lens/cataract has not been removed) patients over age of 30yo.
3. HVF: Humphrey Visual Field: often clinicians order a dilate HVF with eyelids taped up to rule out underlying tumors or vascular pathology. Also used in CACG (chronic angle closure glaucoma & all glaucoma types.
4. Imaging: a clear HVF defect will require an MRI; most eyeMDs will consult neurology first. First-pass contrast-enhanced 3T MRI may successfully image small superficial cranial arteries, but the sensitivity and specificity of this technique in GCA is unproven
5. Diagnostic tools and laboratory tests: should be directed and based on the patient’s medical history and physical exam. Some MDs recommend: platelet count, coagulogram (PT Protime (INR); PTT PTT; PLT Platelet Count; FIBR Fibrinogen), homocysteine and protein S (optional). Some recommend: Tourniquet or capillary-fragility test (Rumpel-Leede test/ Hess test). Optical coherence tomography, retinal oximetry, scanning laser ophthalmoscopic angiography, Doppler studies in order to investigate fundoscopy, visual field examination during the attack are also useful options.
6. What to Look Out for:
1. Brain tumor
2. TÍA/Stroke
3. Associated vascular issues: PMR, ED
4 Reported complications with Retinal Migraine:
– reversible and irreversible central retinal artery occlusion
– retinal infarction
– branch retinal artery occlusion
– retinal hemorrhages and disc edema
– ischemic optic neuropathy
– choroidal ischemia
– dilatation of retinal veins
– vitreous hemorrhage
– retinal pigmentary changes
– stroke
Associations:
Treatment:
There are many treatment options ranging from prevention (ie stay well hydrated, gluten free diets: see Migraine Diet Sheet
https://drcremers.com/2014/02/migraine-diet-recommended-and-not.html?m=1 )
to injectable drugs.
Analgesics and nonsteroidal anti-inflammatory drugs, caffeine, treatment with triptans, ergotamine compounds, may be a favorable option. Triptans and ergotamines, both exert an effect by stimulating the serotonergic receptors in the cerebral and cardiac vasculature. They should be instituted as therapy within 24h of each other.
Prevention
Most migraines, especially retinal migraine attacks, disappear without treatment within one hour or less. People performing activities that require clear vision, when a retinal migraine occurs, need to stop the activity and relax until the vision returns to normal, preferably in a dark or a semi obscure good freshly aerated room. If driving, park on the side of the road and wait for the vision disturbances to pass completely. They should also avoid common migraine triggers and stress, and they should get a regulated sleep and a healthy nutrition: see diet protocol. https://drcremers.com/2014/02/migraine-diet-recommended-and-not.html?m=1
References:
**A study by Taiwanese doctors published in journal Cephalalgia notes an association between ED and migraine so. The researchers analyzed electronic records of one million patients randomly selected out of almost 24 million who are covered by the Taiwan National Health Insurance. They eliminated from this analysis patients with mental illness and they also controlled for hypertension, diabetes, obesity and other condition known to cause ED. Men who suffer from migraines were 1.6 times more likely to have ED. Surprisingly, younger men with migraines, aged 30 to 39 had the highest risk of having erectile dysfunction – they were twice more likely to have ED than men of that age without migraines. The causes for this association are not clear. We do know that patients with chronic pain are more likely to have sexual dysfunction. We also know that migraine patients have impaired regulation of their brain blood vessels, so it is possible that penile blood vessels are also affected.
References:
1.
Migraine and erectile dysfunction: evidence from a population-based case-control study
Corresponding author
Deborah I. Friedman, MD
Departments of Ophthalmology and Neurology, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Box 659, Rochester, NY 14642, USA.
E-mail: deborah_friedman@urmc.rochester.edu
Current Pain and Headache Reports 2008, 12:296–304 Current Medicine Group LLC ISSN 1531-3433
Introduction
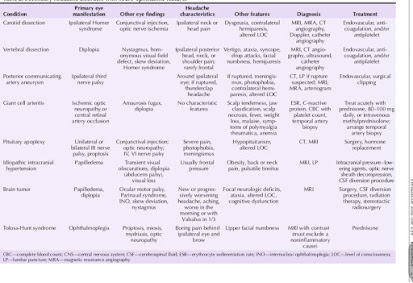
A wide differential diagnosis applies when evaluating a patient with neuro-ophthalmic symptoms and head- ache or periorbital pain. Considerations in the triage of these patients include 1) primary versus secondary headache disorder; 2) ocular or orbital condition ver- sus neurologic disease; 3) extracranial or intracranial process; and 4) the urgency of diagnosis and treatment. Similarly, patients’ interpretation of their condition may lead them to seek medical care from a primary care physician, ophthalmologist, neurologist, neurosurgeon, or in the emergency department. Awareness of these various conditions is essential to achieve the correct diagnosis and management.
Primary Headache Disorders
with Ophthalmic Manifestations
Migraine
Many of the primary headache disorders have neuro-oph- thalmic manifestations. Positive, negative, autonomic, or efferent symptoms and signs are associated with migraine (Table 1). Positive and negative symptoms are part of migraine aura, whereas autonomic and efferent symptoms often occur before or during the headache phase [1–6].
Trigeminal autonomic cephalgias
The trigeminal autonomic cephalgias (TACs) are character- ized by unilateral pain in the distribution of the ophthalmic division of the trigeminal nerve and cranial autonomic acti- vation. Cluster headache is the most common TAC; SUNCT (short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing) and SUNA (short-last- ing unilateral neuralgiform headache attacks with cranial autonomic symptoms) occur infrequently. Autonomic mani- festations of TACs referable to the eye are lacrimation, conjunctival injection, Horner syndrome, and eyelid edema.
Cluster headache
Cluster headache is the most common TAC. It generally occurs in men, producing unilateral attacks of severe pain lasting 15 to 180 minutes. The pain is centered around the eye or the temporal region. The attacks may occur more than once daily, often awakening the patient from sleep at the same time each night. Alcohol and nitrites are common triggers. Most patients with cluster headaches become rest- less or violent during the attacks and prefer to pace or move around while the pain is present. Cluster periods may last from weeks to months and tend to be seasonal and episodic, but occasionally they are chronic, even from the onset.
The autonomic features of cluster headaches affect the ipsilateral eye. During the cluster attack, there is fre- quently a postganglionic Horner syndrome, with ptosis, miosis, and anisocoria that is greater in dim lighting. In some patients, the Horner syndrome may be permanent; it sometimes predates the onset of cluster headaches by years. Patients may also experience conjunctival injection, eyelid edema, and lacrimation, which may be projectile. Other autonomic symptoms include nasal congestion or rhinorrhea, and forehead/facial diaphoresis.
Paroxysmal hemicrania
Paroxysmal hemicrania (PH) is often difficult to dis- tinguish from cluster headache because the site and associated autonomic phenomena are very similar. It usually begins in adulthood but may present at any age. Unlike cluster headache, it affects women more often than men. The pain distribution is the same in both conditions, located in the ophthalmic division of the trigeminal nerve. It is described as severe, stabbing, throbbing, or boring, increasing in intensity over a period of 1 to 40 minutes. Individual episodes are side-locked, although the pain may change sides or occasionally be bilateral or occipital. The episodes are generally shorter than cluster headaches, last- ing 2 to 120 minutes. The spells tend to be more frequent than cluster headaches, often occurring more than five times daily. Another characteristic distinguishing cluster headache from PH is the patient’s response to pain. Most patients with PH lie down, sit quietly, or hold their head during an attack. Attacks may be precipitated by head movement or alcohol and may awaken the patient from sleep. Occasionally PH is associated with aura, migraine headache, or trigeminal neuralgia.
PH may be chronic or episodic. Episodic PH occurs in periods lasting 7 days to 1 year, separated by pain-free intervals of 1 month or more. Chronic PH persists for more than 1 year without remission, or has remissions lasting less than 1 month. Most patients with chronic PH evolve de novo, but there may be a transition from episodic to chronic PH.
Ophthalmic features of PH include an ipsilateral Horner syndrome (ptosis and miosis), as well as the parasympa- thetic nervous system features of lacrimation, conjunctival injection, and eyelid edema. Nasal congestion and rhi- norrhea may occur. Many patients also have migrainous symptoms, such as photophobia, phonophobia, nausea, or vomiting.
PH is exquisitely sensitive to indomethacin; the effect is so predictable that indomethacin responsiveness is included in the diagnostic criteria for the disorder [5]. Thus, when cluster headache is suspected, a trial of indo- methacin is indicated as a “diagnostic test.” Oral dosages of 25 to 75 mg three times daily are used during an attack period. Infrequently, patients not responding to indo- methacin are successfully treated with aspirin, celecoxib, or acetazolamide. If indomethacin is not effective, cluster headache, migraine, or secondary causes of episodic hemi- crania should be considered. Rarely, PH may be mimicked by a tumor in the frontal lobe, cavernous sinus area, or sella. Increased intracranial pressure (ICP) and colla- gen vascular disease uncommonly produce short-lasting unilateral headache resembling PH.
SUNCT and SUNA
SUNCT and SUNA are the rarest of the TACs. SUNCT episodes consist of paroxysmal severe, unilateral pain last- ing 5 to 250 seconds, with a maximum duration of 2 hours [7]. The attack frequency varies from six to 100 daily, with a mean of 28 attacks per day. The attacks may occur so frequently that the pain seems to be constant. As in PH, individual attacks may be precipitated by neck movement.
The autonomic features of SUNCT are similar to clus- ter headache and PH, including prominent conjunctival injection, tearing, and eyelid edema. There may be fore- head diaphoresis and nasal congestion. SUNA is similar to SUNCT, but the attacks last between 2 seconds and 10 minutes, with an attack frequency of one or more per day.
The differential diagnosis of SUNCT includes poste- rior fossa abnormalities, such as arteriovenous and other structural malformations, and HIV/AIDS. MRI is war- ranted to exclude a secondary cause.
Of all the primary trigeminal cephalgias, SUNCT is the most difficult to treat. The best responses are reported with topiramate, lamotrigine, clomiphene citrate, and car- bamazepine [8,9]. SUNCT often worsens with prednisone
and verapamil. Surgical treatments, such as retrogasserian glycerol rhizolysis, gamma knife radiosurgery of the tri- geminal root exit zone, and microvascular decompression surgery, are not consistently effective, and neural stimula- tors are being explored.
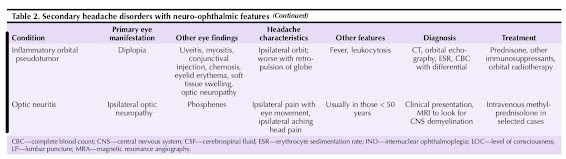
Other Causes of Headache and Periocular Pain Table 2 outlines other causes of headache and peri- ocular pain.
Orbital and ocular etiologies
Keratitis sicca (dry eye)
Dry eye a very common disorder, affecting 10% to 15% of adults. It is associated with tear-deficient states, such as Sjögren syndrome and lacrimal disease, as well as excessive tear evaporation. Other common conditions associated with dry eye are thyroid eye disease that affects the lacrimal gland and also produces corneal exposure, and the decreased blink rate of Parkinsonian disorders. Because the cornea is richly innervated by the ophthalmic division of the trigeminal nerve, corneal surface dryness may be painful. Burning, blurred vision, and photopho- bia commonly occur. Other symptoms of dry eye include monocular diplopia or polyopia, a foreign body sensation in the eye, eye irritation, redness, and tearing. Severe dry eye may cause corneal damage.
Dry eye may simulate headache, and medications used to treat headaches may cause or worsen pre-existing dry eye. Chronic use of medications with anticholinergic side effects, such as tricyclic antidepressants, propranolol, phe- nothiazines, metoclopramide, and muscle antispasmodics, decreases tear production. Positive airway pressure devices used to treat obstructive sleep apnea also contribute to dry eyes; applying a lubricating ointment at bedtime may be helpful for these patients.
Treatment may include lubricating drops (artificial tears), lubricating ointments at bedtime, reversible or per- manent occlusion of the outflow tear ducts, and topical immunosuppressant agents.
Trochlear pain
Trochleitis produces pain in the superomedial part of the orbit that is often exacerbated with eye movement, when the superior oblique tendon apposes the trochlear sling [10]. Some patients experience diplopia and erythema near the superior oblique insertion [11]. The pain is reproduced by palpating the trochlear region. The pain arising from the trochlear area may also trigger the pain of migraine or tension-type headache. It is presumed to be inflammatory in origin and is treated with a local injection of lidocaine or corticosteroids [10].
Angle-closure glaucoma
Angle-closure glaucoma is produced by mechanisms that push the iris forward from behind or pull the iris
forward into contact with the trabecular meshwork. Sudden blockage of the trabecular meshwork by the iris prevents drainage of the aqueous humor, producing a rapid increase in intraocular pressure. Acute angle- closure glaucoma is characterized by pain, blurred vision, rainbow-colored halos around lights, nausea, and vomiting. Examinations reveal high intraocular pressure, a mid-dilated and sluggishly reactive pupil, corneal edema (“steamy” cornea), dilated conjunctival blood vessels, a shallow anterior chamber, and mild aqueous inflammatory changes.
Angle-closure glaucoma usually occurs in the setting of shallow anterior angles or a plateau iris (anteriorly positioned ciliary processes), but may occur under certain conditions in patients with normal ocular anatomy. Sulfa derivatives, such as topiramate and acetazolamide, may precipitate angle-closure glaucoma (usually bilateral and simultaneous) as an idiosyncratic reaction. Anticholinergics and antidepressants may produce attacks in predisposed individuals. Prompt treatment is necessary to prevent per- manent visual loss and pupillary deformity.
Subacute angle-closure glaucoma may mimic migraine, producing episodes of ocular or periocular pain, halos, and blurred vision [12]. The attacks are often precipitated by rapid miosis, such as when emerging from a dark the- ater into daylight. Sleep-induced mydriasis relieves the symptoms, which may be mistaken for the sleep-induced pain relief of migraine.
Inflammatory orbital disease
Many conditions produce inflammatory orbital disease, and the differential diagnosis includes systemic disorders, neoplasm, congenital malformations, infectious disease, and trauma [11]. Orbital involvement from idiopathic inflammation may involve any of the orbital structures, including the extraocular muscles (myositis), sclera (scleri- tis, episcleritis), aqueous or vitreous (uveitis), lacrimal gland (dacryoadenitis), and uncommonly, the retinae or optic nerves. Idiopathic inflammation, also termed inflammatory orbital pseudotumor, produces unilateral or bilateral symp- toms of diplopia, pain, proptosis, conjunctival injection, photophobia, and periorbital edema [13•]. The pain may be severe and is exacerbated by manually retropulsing the globe under closed eyelids. The diagnosis is usually made clinically after excluding systemic causes. Corticosteroids are the mainstay of therapy; other immunosuppressive agents and radiotherapy are occasionally used when corti- costeroids are ineffective.
Tolosa-Hunt syndrome, characterized by painful oph- thalmoplegia, is a form of idiopathic inflammation of the cavernous sinus or superior orbital fissure. It rarely extends into the orbit or affects the lower divisions of the trigeminal nerve. It may occur at any age and responds dramatically to corticosteroids. Tolosa-Hunt syndrome is uncommon and is a diagnosis of exclusion, as lymphoma, metastatic dis- ease, sarcoidosis, Wegener’s granulomatosis, vasculitis, and infectious conditions produce an identical phenotype and improve with corticosteroids [14].
Vascular disorders
Cervical arterial dissection
Cervical arterial dissections involving the internal carotid arteries and the vertebral arteries occur at regions where the arteries are mobile and not fixed to other arteries or bony structures. These segments are vulnerable to tear- ing and are usually unilateral. They begin with a tear in the media that leads to longitudinal intramural dis- section proximally and distally. If the intima is torn, partially coagulated blood enters the arterial lumen, expanding the arterial wall, activating the coagulation cascade in the vascular endothelium, and producing an intraluminal thrombus. A dissection originating in the intima produces a flap, creating true and false lumens. An aneurysmal outpouching of the arterial wall dissec- tion plane causes a pseudoaneurysm. Rupture through the adventitia intracranially leads to subarachnoid hem- orrhage [15••]. Pain is present in nearly all patients with cervical and intracranial arterial dissections.
Congenital and acquired connective tissue disorders, such as Marfan syndrome, Ehlers-Danlos syndrome, and fibromuscular dysplasia, are predisposing factors. A history of migraine is common among patients with dissection. There may be antecedent trauma or a history of sudden or unusual movements stretching the arteries. Often an underlying cause is not found.
Carotid artery dissection
The most common symptoms of carotid artery dis- section are pain in the ipsilateral face, neck, or head; transient monocular visual loss; contralateral weakness or numbness; stroke; Horner syndrome (ipsilateral ptosis and miosis); and pulsatile tinnitus. Lower cranial nerve symptoms include dysphagia, hoarseness, dysgeusia, and sternocleidomastoid weakness. The pain may precede the focal neurologic symptoms by days to weeks. Extracranial dissections usually produce ipsilateral trigeminal pain and a Horner syndrome, which may be indistinguishable from an attack of cluster headache.
CT angiography, MRI, B-mode ultrasound, and magnetic resonance angiography (MRA) are effective for imaging the affected arterial walls and lumen [16]. MRA is more useful in detecting carotid than vertebral dissection. Catheter angiography may be used. There are no evidence- based guidelines for treatment. Endovascular procedures, such as intra-arterial thrombolysis or clot extraction, stenting, and angioplasty, are emerging therapies. Antico- agulation and antiplatelet therapies are also used, although there is no consensus regarding their use [17].
Vertebral artery dissection
The pain of cervical vertebral artery dissection often precedes the neurologic symptoms and is localized to the
ipsilateral trapezius, posterior neck, occiput, or cervical nerve roots [15••]. Occasionally, the headache is frontal [18]. There may be transient ischemic attacks, stroke, or no neurologic symptoms. Spinal cord infarction rarely occurs. Intracranial involvement causes infarction or subarachnoid hemorrhage. The most common transient symptoms are dizziness, diplopia, lateropulsion, stag- gering, and dysarthria. Strokes may produce visual field defects, nystagmus, Horner syndrome, corneal anesthesia, and ptosis [18].
Intracranial aneurysms
Most aneurysms (85%) originate from the internal carotid artery and its branches, whereas the rest are infratento- rial. The risk of rupture increases with age, location, size of the aneurysm, and presence of symptoms. Intracavern- ous, carotid-ophthalmic, and posterior communicating artery aneurysms often cause headache and visual symp- toms as initial features [19]. They are diagnosed using CT angiography, MRI and MRA, and catheter angiography. Treatment modalities include neurosurgical and endo- vascular procedures [20].
Intracavernous aneurysms may not cause symptoms until they are quite large. They are sometimes discov- ered incidentally when evaluating a patient for another aneurysm. Because they are encased by bone, they rarely rupture, but may present with a spontaneous cavernous- carotid fistula [21]. They generally affect older women, are associated with hypertension, and may be bilateral. The symptoms are produced by compression of surround- ing structures, most commonly the abducens nerve. The oculomotor and trochlear nerve may also be affected. Common symptoms are diplopia and pain. Pain, present in approximately 90% of patients, consists of headache, retro-orbital pain, or trigeminal pain. The examination shows one or more ocular motor nerve palsies, Horner syndrome, and corneal anesthesia. Involvement of the dis- tal portion of the intracavernous carotid produces visual loss with the associated features of an optic neuropathy. If treatment is needed, these aneurysms are generally approached endovascularly because of their location.
An aneurysm at the bifurcation of the carotid and posterior communicating arteries may compress the ocu- lomotor nerve as it traverses the subarachnoid space. The pupil is usually involved, and the oculomotor palsy may be otherwise incomplete. The pain associated with an aneurysmal oculomotor palsy is generally localized to the ipsilateral eye area. Prompt diagnosis is needed to prevent rupture. Once rupture occurs, there may be altered level of consciousness, meningismus, photophobia, or mani- festations of brain herniation. Posterior communicating artery aneurysms are treated with surgical clipping or endovascular procedures.
Carotid-ophthalmic artery aneurysms arise from the junction of the internal carotid artery and the ophthalmic artery and account for approximately 5% of all intracranial aneurysms. True ophthalmic artery aneurysms are rare. They are more frequent in women, most commonly left-sided, and often associated with other aneurysms. The most common symptom is monocular visual loss, which may be slowly progressive or acute and painful. An optic neuropathy is the most frequent sign, although posterior or superior expansion may produce an optic chiasmal or optic tract syndrome. Medial expansion causes bilateral optic neuropathies, often with asymmetric involvement. These aneurysms may enlarge to produce ocular motor palsies and may rupture.
Basilar artery aneurysms usually rupture before they cause focal neurologic deficits. Both ruptured and unruptured basilar artery aneurysms may cause head- aches that are generally suboccipital but may be orbital or periorbital. The headaches are often aggravated by head motion. Neuro-ophthalmic signs include abducens paresis, horizontal gaze paresis, nystagmus, oculomotor nerve palsy, dorsal midbrain (Parinaud) syndrome, and internuclear ophthalmoplegia. If the aneurysm is large enough to obstruct the aqueduct, papilledema and visual loss may occur from ICP. Treatment of basilar artery aneurysms is challenging because of their location and usually approached endovascularly.
Systemic disorders
Giant cell arteritis
Giant cell arteritis (GCA), or temporal arteritis, is a systemic vasculitis with protean manifestations. Thus, patients with GCA may seek care from a primary care physician, dentist, ophthalmologist, neurologist, rheu- matologist, dermatologist, or cardiologist, depending on their symptoms. Because it can produce bilateral, irre- versible blindness, a high index of suspicion is needed for prompt diagnosis and treatment [22••]. Moreover, there is no single diagnostic test that accurately defines the dis- order [23••]. The incidence increases with age, ranging from 1.4 per 100,000 between ages 50 and 59 years to 29.6 per 100,000 between ages 70 and 79 years [24••].
The headache of GCA is not specific; GCA must be considered in any patient age 60 years or older who develops a new headache. The pain may be global, hemi- cranial, or bifrontal, similar in character to migraine or tension-type headache pain. Other patients experience brief stabbing head pain. There is often accompanying scalp tenderness. Pain and weakness (claudication) of the jaw while chewing is highly suggestive of GCA and is often misdiagnosed as temporomandibular joint dys- function. Other systemic symptoms include weight loss, fever, myalgias, arthralgias, scalp necrosis, depression, malaise, and fatigue. Polymyalgia rheumatica is present in approximately 50% of cases and may represent a mild form of GCA. Stroke, myocardial infarction, and bowel infarction are infrequent complications.
Unfortunately, approximately one third of patients with GCA evaluated by ophthalmologists and neuro-
ophthalmologists present with visual loss in the absence of other systemic symptoms, so-called “occult” GCA. Anterior ischemic optic neuropathy (AION) associated with GCA may produce severe loss of visual acuity and visual field, with pallid swelling of the optic nerve. There may be evidence of retinal ischemia. Rapid progression to involve the fellow eye or bilateral, simultaneous AION commonly occurs with arteritic AION. There is also a nonarteritic form of AION, which is much more common than the arteritic variety and may be difficult to distin- guish clinically from GCA. Fluorescein angiography may be helpful in this circumstance, showing delayed cho- roidal filling time in arteritic AION. Some patients with GCA experience amaurosis fugax or transient diplopia prior to developing permanent visual loss. Central retinal artery occlusion is responsible for about 10% of GCA- associated visual loss.
The diagnosis of GCA is supported by several labora- tory tests, including an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein, thrombocytosis, anemia of chronic disease, and increased fibrinogen levels. How- ever, all of these tests may be normal in GCA, and none is specific for GCA. The sensitivity of an elevated ESR is 84.9%, an elevated C-reactive protein is 97.5% sensitive, and the sensitivity increases to 99.2% when both tests are abnormal [25]. First-pass contrast-enhanced 3T MRI may successfully image small superficial cranial arteries, but the sensitivity and specificity of this technique in GCA is unproven [26].
The “gold standard” for diagnosis is a temporal artery biopsy, showing narrowing or thrombosis of the arterial lumen, inflammation with or without giant cells, and disruption of the internal elastic lamina. A 2-cm–long specimen with 1-mm serial-sectioned histology prepara- tions is recommended, as skip lesions occur. The overall sensitivity of a temporal artery biopsy is 87.1% [25], and bilateral biopsies do not increase the yield significantly. Many physicians dismiss the biopsy as unnecessary and make a diagnosis solely on clinical grounds. I disagree with this approach, as the diagnosis may be questioned later after the patient experiences complications from cor- ticosteroids. Moreover, GCA requires daily corticosteroid treatment for upwards of a year; one should be as certain of the diagnosis as possible before committing a patient to the treatment.
The philosophy of suspected GCA, particularly if there is visual loss, optic disc edema, or amaurosis, is to “treat first and ask questions later.” The gener- ally accepted window for performing a temporal artery biopsy after starting treatment is 10 days, although evidence of inflammation may persist after longer time periods. There are no evidence-based dosing guidelines, but high-dose corticosteroids (80–100 mg of prednisone) should be started expeditiously. In the setting of acute, unilateral, arteritic AION, one may elect to adminis- ter intravenous corticosteroids to prevent visual loss in the other eye, although progression may occur despite intravenous therapy. Corticosteroids are the mainstay of treatment; other immunosuppressant agents have not been proven effective for corticosteroid substitution, and their role as steroid-sparing agents is limited. Neuro-ophthalmologists tend to taper the steroids more slowly and use a high maintenance dose of prednisone (20 mg daily vs 10–15 mg daily), compared with rheumatologists. I suspect that this reflects differences in patient populations between specialties; patients with visual loss tend to have more severe disease. The systemic symptoms remit quickly with prednisone treatment, but the visual prog- nosis is quite poor in arteritic AION.
Idiopathic intracranial hypertension and increased ICP
Headaches associated with bilateral optic disc edema indicate increased ICP, which may be caused by tumors, infarction, inflammation, and congenital anomalies. Idio- pathic intracranial hypertension (IIH) is characterized by increased ICP without a mass, ventriculomegaly, or other identifiable cause. It most commonly affects obese women of childbearing age, although it may occur in chil- dren and men. The most common symptom is headache, present in over 90% of patients. The headache is non- specific and may be retro-orbital, bifrontal, unilateral, or posteriorly located. The pain may be throbbing or steady, constant or intermittent, and is generally severe. There may be accompanying photophobia, phonophobia, nau- sea, and vomiting. Some patients, particularly children, have more prominent neck and back pain than headache [27••]. Brief episodes of complete or partial visual loss, termed transient visual obscurations, are often precipi- tated by postural change and reflect papilledema. Other symptoms include visual loss, pulsatile tinnitus, diplopia, radicular pain, and ataxia. The diagnostic hallmark of IIH is papilledema, although it is not universally present and may be asymmetrical.
IIH is diagnosed by excluding a mass lesion or venous sinus thrombosis, and demonstrating an increased cere- brospinal fluid (CSF) pressure with otherwise normal CSF contents. Thus, MRI, magnetic resonance venography, and a diagnostic lumbar puncture should be performed on all patients. Detailed documentation of the visual status is necessary, including perimetry. One must also exclude a secondary cause [28••].
The goal of treatment is to preserve vision. With mini- mal or mild visual impairment, medication is usually an appropriate first-line treatment. Medical therapy includes decreasing salt intake, acetazolamide, and headache ther- apy. If acetazolamide cannot be tolerated, furosemide or other diuretics are used. Retrospective studies suggest that modest weight loss (5% to 6% of body weight) may hasten the resolution of papilledema. Preventive headache therapy is often required, and medication overuse is possible in these patients; topiramate has the advantages of mild carbonic anhydrase activity and possible weight loss.
When vision is impaired or if the visual status rap- idly deteriorates, surgical options include optic nerve sheath fenestration and CSF diversion procedures (shunting). The role of venous sinus stenting for IIH is controversial [29,30].
Conclusions
The diagnosis and management of conditions producing headache with ocular or neuro-ophthalmologic manifes- tations is challenging. Many of these conditions require a multidisciplinary approach. Recognizing ocular condi- tions and secondary disorders, and obtaining appropriate referral and testing, results in prompt intervention that may preserve vision or save the patient’s life.
Disclosure
No potential conflict of interest relevant to this article was reported.
References and Recommended Reading Papers of particular interest, published recently,
have been highlighted as: • Ofimportance
•• Of major importance
1. Leone M, Proicetti Cecchini A, Mea E, et al.: Functional neuroimaging and headache pathophysiology: new findings and new prospects. J Neurol Sci 2007, 28:S108–S113.
2. Liu GT, Schatz NJ, Galetta SL, et al.: Persistent positive visual phenomena in migraine. Neurology 1995, 45:664– 668.
3. Grosberg BM, Solomon S, Lipton RB: Retinal migraine. Curr Pain Headache Rep 2005, 9:268–271.
4. Jacobson DM: Benign episodic unilateral mydriasis. Clinical characteristics. Ophthalmology 1995, 102:1623–1627.
5. Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders. Cephalalgia 2004, 24(Suppl 1):1–151.
6. McMillan HJ, Keene DL, Jacob P, Humphreys P: Ophthal- moplegic migraine: inflammatory neuropathy with secondary migraine? Can J Neurol Sci 2007, 34:349–355.
7. Williams MH, Broadley SA: SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci 2008, 15:526–534.
8. Rozen TD, Saper JR, Sheftell FD, Dodick DW: Clomiphene citrate as a new treatment for SUNCT (hormonal manipula- tion for hypothalamic influenced trigeminal autonomic cephalgias). Headache 2005, 45:754–756.
9. May A, Leone M, Áfra A, et al.: EFNS guidelines on
the treatment of cluster headache and other trigeminal autonomic cephalgias. Eur J Neurol 2006, 13:1066–1077.
10. Pareja JA, Sánchez del Río M: Primary trochlear headache and other trochlear painful disorders. Curr Pain Headache Rep 2006, 10:316–320.
11. Gordon LK: Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye 2006, 20:1196–1206.
12. Gilbert ME, Friedman D: Migraine and anisocoria. Surv Ophthalmol 2007, 52:209–212.
13.• Bhatti MT: Orbital syndromes. Semin Ophthalmol 2007, 2 7 : 2 6 9 – 2 8 7.
A comprehensive review of orbital anatomy and clinical manifestations of orbital syndromes, with a discussion of various orbital disorders.
14. Gladstone JP: An approach to the patient with painful ophthalmoplegia, with a focus on Tolosa-Hunt syndrome. Curr Pain Headache Rep 2007, 6:129–147.
15.•• Caplan LR: Dissections of brain-supplying arteries. Nat Clin Pract Neurol 2008, 4:34–42.
This article incorporates Dr. Caplan’s extensive experience to describe the anatomy and physiology of cervical dissections, their clinical manifestations, and treatment.
16. Flis CM, Jäger HR, Sidhu PS: Carotid and vertebral artery
dissections: clinical aspects, imaging features and endovas-
cular treatment. Eur Radiol 2007, 17:820–834.
17. Engelter ST, Brandt T, Debette S, et al.: Antiplatelets versus
anticoagulation in cervical artery dissection. Stroke 2007,
38:2605–2611.
18. Umasankar U, Carroll TJ, Famuboni A, et al.: Vertebral
artery dissection: not a rare cause of stroke in the young.
Age Ageing 2008, 37:345–346.
19. Gilbert ME, Sergott RC: Intracranial aneurysms. Curr Opin
Ophthalmol 2006, 17:513–518.
20. Queseshi AI, Janardhan V, Hanel RA, Lanzino G: Compari-
son of endovascular and surgical treatments for intracranial aneurysms: an evidence-based review. Lancet Neurol 2007, 6:816–825.
21. Stiebel-Kalish H, Kalish Y, Setton A, et al.: Presentation, natural history, and management of carotid cavernous aneurysms. Neurosurgery 2005, 57:850–857.
22.•• Danesh-Meyer HV, Savino PJ: Giant cell arteritis. Curr Opin Ophthalmol 2007, 18:443–449.
Danesh-Meyer and Savino, both neuro-ophthalmologists, present a very practical approach to GCA.
23.•• Melson MR, Weyland CM, Newman NJ, Biousse V: The
diagnosis of giant cell arteritis. Rev Neurol Dis 2007,
4:128–142.
Very detailed article written from the neuro-ophthalmic perspective. Neuro-ophthalmologists have the greatest experience with patients who sustain visual loss, one of the most feared complications of GCA.
24.•• Schwedt TJ, Dodick DW, Caselli RJ: Giant cell arteritis. Curr Pain Headache Rep 2006, 10:415–420.
The authors approach GCA from the perspective of headache medicine specialists.
25. Niederkohr RD, Levin LA: Management of the patient with
suspected temporal arteritis: a decision-analytic approach.
Ophthalmology 2005, 112:744–756.
26. Markl M, Uhl M, Wieben O, et al.: High resolution 3T
MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging 2007, 24:423–427.
27.•• Rangwalla LM, Liu GT: Pediatric idiopathic intracranial hypertension. Surv Ophthalmol 2007, 52:597–617.
The first truly definitive article on pediatric IIH, including new criteria for diagnosis in children.
28.•• Friedman DI: Idiopathic intracranial hypertension. Curr
Pain Headache Rep 2007, 11:62–68.
A comprehensive review of the diagnosis and treatment of IIH, focusing primarily on adolescents and adults.
29. Donnet A, Metellus P, Levrier O, et al.: Endovascular treat-
ment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology 2008, 70:641–647.
30. Friedman DI: Cerebral venous pressure, gastric bypass surgery and dural venous sinus stenting in idiopathic intra- cranial hypertension. J Neuro-ophthalmol 2006, 26:61–64.