This is an interesting article noting that the funds used via Proposition 71, which created the California Institute for Regenerative Medicine, or CIRM has helped find stem cell cures for a rare genetic condition called Severe Combined Immunodeficiency, or SCID, also known as “bubble baby” disease, which severely impairs the immune system and increases the risk of death in affected children.
The National Institutes of Health notes approximately 40 to 100 children in the U.S. each year are diagnosed with the ailment.
A UCLA team, funded by CIRM, genetically modified children’s blood stem cells to correct the SCID mutation: 40 children have been cured.
But note: No CIRM-funded stem cell treatment has yet to be approved by the FDA for general use yet. See Reference 2. It takes a long time even with good proof of improvement to get FDA approved.
Reference 1:
jCyte Shares Encouraging Update on Clinical Trial for Retinitis Pigmentosa
Stepping out of the darkness into light. That’s how patients are describing their experience after participating in a CIRM-funded clinical trial targeting a rare form of vision loss called retinitis pigmentosa (RP). jCyte, the company conducting the trial, announced 12 month results for its candidate stem cell-based treatment for RP.
RP is a genetic disorder that affects approximately 1 in 40,000 individuals and 1.5 million people globally. It causes the destruction of the light-sensing cells at the back of the eye called photoreceptors. Patients experience symptoms of vision loss starting in their teenage years and eventually become legally blind by middle age. While there is no cure for RP, there is hope that stem cell-based therapies could slow its progression in patients.
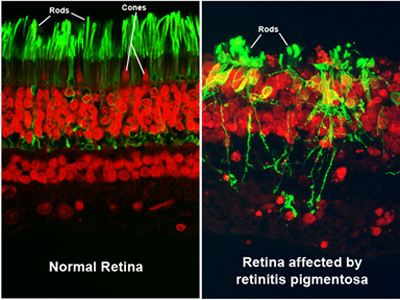
Photoreceptors look healthy in a normal retina (left). Cells are damaged in the retina of an RP patient (right). (Source National Eye Institute)
jCyte is one of the leaders in developing cell-based therapies for RP. The company, which was founded by UC Irvine scientists led by Dr. Henry Klassen, is testing a product called jCell, which is composed of pluripotent stem cell-derived progenitor cells that develop into photoreceptors. When transplanted into the back of the eye, they are believed to release growth factors that prevent further damage to the surviving cells in the retina. They also can integrate into the patient’s retina and develop into new photoreceptor cells to improve a patient’s vision.
Positive Results
At the Annual Ophthalmology Innovation Summit in November, jCyte announced results from its Phase 1/2a trial, which was a 12-month study testing two different doses of transplanted cells in 28 patients. The company reported a “favorable safety profile and indications of potential benefit” to patient vision.
The patients received a single injection of cells in their worst eye and their visual acuity (how well they can see) was then compared between the treated and untreated eye. Patients who received the lower dose of 0.5 million cells were able to see one extra letter on an eye chart with their treated eye compared to their untreated eye while patients that received the larger dose of 3 million cells were able to read 9 more letters. Importantly, none of the patients experienced any significant side effects from the treatment.
According to the company’s news release, “patient feedback was particularly encouraging. Many reported improved vision, including increased sensitivity to light, improved color discrimination and reading ability and better mobility. In addition, 22 of the 28 patients have been treated in their other eye as part of a follow-on extension study.”
One of these patients is Rosie Barrero. She spoke to us earlier this year about how the jCyte trial has not only improved her vision but has also given her hope. You can watch her video below.
Next Steps
These results suggest that the jCell therapy is safe (at least at the one year mark) to use in patients and that larger doses of jCell are more effective at improving vision in patients. jCyte CEO, Paul Bresge commented on the trial’s positive results:
 Paul Bresge“We are very encouraged by these results. Currently, there are no effective therapies to offer patients with RP. We are moving forward as quickly as possible to remedy that. The feedback we’ve received from trial participants has been remarkable. We look forward to moving through the regulatory process and bringing this easily-administered potential therapy to patients worldwide.”
Paul Bresge“We are very encouraged by these results. Currently, there are no effective therapies to offer patients with RP. We are moving forward as quickly as possible to remedy that. The feedback we’ve received from trial participants has been remarkable. We look forward to moving through the regulatory process and bringing this easily-administered potential therapy to patients worldwide.”
Bresge and his company will be able to navigate jCell through the regulatory process more smoothly with the product’s recent Regenerative Medicine Advanced Therapy (RMAT) designation from the US Food and Drug Administration (FDA). The FDA grants RMAT to regenerative medicine therapies for serious diseases that have shown promise in early-stage clinical trials. The designation allows therapies to receive expedited review as they navigate their way towards commercialization.
jCyte is now evaluating the safety and efficacy of jCell in a Phase2b trial in a larger group of up to 85 patients. CIRM is also funding this trial and you can read more about it on our website.
January 25, 2018
Will State Voters Continue To Pour Money Into Stem Cell Research?

Courtesy of Alysia Padilla-Vaccaro
The year was 2004, and according to certain TV ads in California, great medical breakthroughs might be just around the corner.
In these political ads, celebrities Michael J. Fox and Christopher Reeve, both facing serious, chronic conditions, touted the promise of stem cell research, which they believed could lead to a plethora of cures for life-threatening diseases.
The ads ran in support of Proposition 71, a $3 billion California bond measure that would create the first state-funded stem cell agency in the nation. Three years earlier, the George W. Bush administration had issued rules to limit federal funding of the use of stem cells obtained from human embryos.
California voters easily passed Proposition 71 — 59 percent to 41 — and the California Institute for Regenerative Medicine, or CIRM, was born. Its mission: to fund and accelerate stem-cell-related treatments.
Today, 14 years and billions of dollars later, that California agency is running out of money, and backers of stem cell research plan to ask voters in the state to pony up for round two. The projected ask this time: $5 billion, in a measure the backers hope to place on the California ballot in 2020.
For voters this time, there will be one major question, says Zev Yaroslavsky, a former member of the Board of Supervisors for Los Angeles County, and now a specialist in state politics and government at UCLA:
“The public will want to know,” he says, “what they’ve gotten for their money.”
For voters this time, there will be one major question, says Zev Yaroslavsky, a former member of the Board of Supervisors for Los Angeles County, and now a specialist in state politics and government at UCLA:
“The public will want to know,” he says, “what they’ve gotten for their money.”
Across the U.S., nearly a dozen states have followed California’s example in launching their own stem cell initiatives, and many more will be paying close attention to what happens in that bellwether state.
“We have been most definitely influenced and inspired by CIRM,” says Dr. Charles Murry, a cardiovascular pathologist with the University of Washington’s Institute for Stem Cell and Regenerative Medicine. Washington is among the most recent states to start directly funding regenerative medicine research.
“When I talked to legislators about this,” Murry says, “the fact that other states have stepped up and done this in such a big way — that helped a lot. CIRM definitely has been a trailblazer for the rest of us.”
Robert Klein, who spearheaded the original 2004 California ballot measure and served as chairman on CIRM’s first board, still heads the advocacy group, Americans For Cures, that pushed Proposition 71. Medical science isn’t exactly his field — he’s president of a Palo Alto-based real estate development firm — but he got involved in stem cell funding because of his son’s Type 1 diabetes, a chronic condition. Klein says re-funding the stem cell agency is not just a good cause, but also good business for California.
“It has been a creator of jobs, and the state benefits from taxes by attracting research centers here,” he says.
In 2012, an independent study commissioned by CIRM to estimate the economic impact of the agency’s grants and matching funds through 2014 suggested the program would result in, on average, more than 4,000 new jobs per year, and $205 million in state tax revenue.
“It has been a creator of jobs, and the state benefits from taxes by attracting research centers here,” he says.
In 2012, an independent study commissioned by CIRM to estimate the economic impact of the agency’s grants and matching funds through 2014 suggested the program would result in, on average, more than 4,000 new jobs per year, and $205 million in state tax revenue.
As for the proposed new funding, Klein says the $5 billion bond cost would be amortized over 40 years, so is not a huge cost compared to other government projects.
“Look, we paid $6.5 billion just to fix the eastern span of the Bay Bridge,” Klein says. “That’s road infrastructure — this is more like [funding] the intellectual infrastructure of California.”
But where are the cures?
California’s ballot initiative struck an emotional chord in 2004, in part because of the high profile cases of actors Reeve and Fox. Reeve, who died in 2004, had been paralyzed by a 1995 injury to his spine in a horse-riding accident; Fox has Parkinson’s, a neurodegenerative disease.
Both men hoped that one day therapies based on stem cell research could bridge or repair broken neural or neuromuscular connections and help them and others who have similar conditions.
Stem cells are undifferentiated, which means they have the ability to be transformed into cells of specific tissues and organs — possibly for use in new therapies that might treat or even cure some diseases.
In Fox’s 30-second spot, he used the word “cures” three times.
In Fox’s 30-second spot, he used the word “cures” three times.
YouTube
So, has CIRM produced any cures?
Five-year-old Evangelina Padilla-Vaccaro, who graces the cover of CIRM’s 2016 annual report, is the example many people cite.
Evangelina was born with a rare genetic condition called Severe Combined Immunodeficiency, or SCID, also known as “bubble baby” disease, which severely impairs the immune system. Most children who have the condition must live in a highly controlled, isolating environment to avoid an infection, which can be lethal.
The National Institutes of Health estimates approximately 40 to 100 children in the U.S. each year are diagnosed with the ailment.
Partially funded by CIRM, a team of UCLA clinical researchers were able to genetically modify Evangelina’s own blood stem cells to correct the SCID mutation.
She and at least 40 other children have been cured, according to CIRM.
Despite this success, the treatment trial for SCID is only in Phase 2 along the lengthy road to FDA approval. CIRM has only two clinical trials in Phase 3: One of these studies is testing a new shunt for kidney dialysis patients that is made out of human tissue and does not have to be replaced; the second trial is testing a treatment that aims to slow down the progression of Lou Gehrig’s disease.
Other promising CIRM-funded therapies include slowing or reversing retinitis pigmentosa, a genetic abnormality that destroys a person’s sight; and injecting stem cells into newly injured spinal trauma patients.
The FDA has made several of these therapies eligible for priority review, by granting them Regenerative Medicine Advanced Therapy, or RMAT, status. Clinical trials and studies in less-advanced stages are ongoing for many other diseases and conditions, including brain cancer, diabetes and HIV.
But the fact remains: Although it could change in the run-up to the election, no CIRM-funded stem cell treatment has yet to be approved by the FDA for general use.



