On a microbiologic level, in 2005, Frank Schirra, MD, reported a DNA receptor study that confirmed the presence of more than 1,500 genes coding for androgen activity in these tissues. 4
Clinical studies
Based on the revelations of the Sullivan research, in 2003, C.G. Connor at the Southern School of Optometry, Memphis, developed and patented a transdermal 3% androgen eyelid preparation specific for the treatment of DED. 6
He found that testosterone when given as an eye drop is effective but “the poor solubility of androgens resulted in considerable irritation and poor patient compliance.”
The transdermal preparation was licensed to an orphan drug company (arGentis Pharmaceuticals), which completed a phase II study. They reported a 68% increase in tear break-up time and a 51% increase in aqueous secretion with a 51% decrease in symptoms. They also had good results with transdermal delivery of progesterone. Neither, however, made it to market.
In 2008, Allergan commissioned a phase I study 4 with the National Institute of Health titled, “The Safety and Efficacy Study of a Testosterone Eye Drop with a Treatment of Meibomian Gland Dysfunction.”
The results after 6 months were significant, with 30% of patients with non-inspissated glands becoming asymptomatic in the androgen group versus only 8% in the control group.
DHEA was the testosterone analog used in that study. DHEA is the primary hormone produced by the adrenal and is a metabolic precursor, but only about 50% is naturally converted into testosterone. The most effective concentration in their study was 0.03%. Allergan submitted another U.S. patent application in 2012 5 and initiated a phase II study, due for completion in 2015.
The phase I study was the impetus for my own mini-office study. The intention, however, was to use an enhanced bioavailable and more tolerable aqueous soluble testosterone eye drop formulation.
Testosterone
Testosterone, when given orally in high doses to overcome first pass hepatic clearance, can risk toxicity. Parenteral dosing is therefore required, either administered daily by a transdermal or transmucosal route, or as a repository intramuscular injection every 2 weeks.
Commercial preparations of testosterone are readily available but have objectionable qualities, such as cost, inconvenience, or discomfort.
The advantage of an eye drop administration route is that it is convenient and avoids the untoward effects from a high systemic androgen load.
Primary-care physicians and gynecologists are hesitant to prescribe systemic doses of testosterone for women because of occasional cosmetically unacceptable viralization effects, such as chin hair growth or acne.
There also is resistance in the medical community to prescribing testosterone supplementation to men because of what many consider an erroneous dogma from the 1940s, based on one inadequate study and despite all recent evidence to the contrary. That assertion was that high testosterone levels enhanced prostate carcinoma growth.
Longitudinal studies have, however, repeatedly and consistently rejected that hypothesis.
I found that Leiter’s Pharmacy, one of the largest ophthalmic compounding pharmacies in the United States, dispensed an androgen eye drop.
They reported to me that it had favorable but inconsistent results but also often caused ocular irritation. It was formulated with DHEA, the metabolic precursor to testosterone in a high concentration as an emulsion. Emulsions have an inherent problem due to their high viscosity, requiring vigorous agitation to maintain concentration consistency and to avoid clogging of the dispenser bottle tip.
I collaborated with them in reformulating the hormone as testosterone and not DHEA, and in a much lower concentration with a novel and well-known aqueous solubilizer, cyclodextrin.
All sterol hormones such as the sex hormones have relatively low surface reactivity and are poorly water or lipid soluble. In an aqueous medium, they must be dispersed as a nanoparticle suspension as an emulsion.
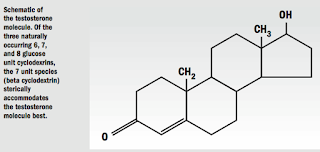
My research revealed an ideal aqueous solubilizing compound for delivery of sterols to the eye, cyclodextrin. This was reported in a Scandinavian ophthalmologic journal in 2002. 7
Cyclodextrin has been known for more than a century and is classified by the USDA as generally recognized as safe for human use. Because of its low cost and availability in recent years, it has experienced broad applications in the food, pharmaceutical, and chemical industries. Potential drug applications of cyclodextrin have been realized and a few ophthalmic eye drops, such as Voltaren, have been formulated with it.
In 2009, I designed my own small in-office, 2-week study enrolling 12 consecutive patients (9 women and 3 men) with significant DED symptoms based on the Allergan Ocular Surface Disease Index. Pre- and post-treatment Schirmer’s and lissamine green staining measurements were obtained.
As a double-blind study, patients were issued two identical-appearing eye drop bottles, one containing only the control vehicle solution and the other, 0.03% testosterone solubilized with hydroxyl propyl beta cyclodextrin.
They were instructed to instill one drop in an eye chosen at random and the other eye drop on the fellow eye and not to cross contaminate.
After 2 weeks, they were re-examined for effects. Ten of the 12 had significant improvement in symptoms and signs.
Leiter’s then broke the code to reveal what the bottles contained. Surprisingly, improvement occurred almost equally in both eyes. I presumed that enough systemic absorption of the therapeutic drop occurred in one eye to affect the control eye.
Treatment Protocol
Although the study was statistically underpowered due to the small sample size, the beneficial results were enough for me to develop a treatment protocol. Leiter’s added this to its dispensing formulary. I decided to also include 0.05% progesterone in the formulation, based on reports also indicating beneficial effects of this hormone in decreasing dry eye symptoms.
I speculate that progesterone is effective because it is converted to testosterone in the sterol pathway and also is converted to a corticosteroid that could afford anti-inflammatory effects. Estrogen was avoided since the Woman’s Health Study revealed that women on estrogen replacement alone have a higher incidence of DED.
For any degree of dry eye, I prescribe the omega-3 fatty acid nutraceutical, eicosapentanoic acid (EPA). The beneficial effects of dietary omega-3 fatty acids in reducing DES symptoms have been reported from multiple studies. 8,9 EPA is the most effective anti-inflammatory omega-3 fatty acid and is present in high concentration in refined fish oils.
In mildly symptomatic patients, omega-3 nutraceuticals, tear substitutes, and environmental modifications may be sufficient to relieve symptoms and not require androgen replacement.
Because a testosterone eye drop is a compounded drug and therefore not usually reimbursed by insurance, I limit prescription to motivated patients with moderate to severe lissamine green staining and significant DED symptoms. I instruct them to instill one drop in each eye and, to affect some transdermal absorption, lightly massage the overflow into the lid margin and palpebral lid area.
Although twice-daily dosing is prescribed, once daily may be effective. I have found that a majority of women in the perimenopause age range of 45-60 years, or earlier, if they have had surgical menopause, have evaporative dry eye and will respond favorably to an androgen eye drop.
As they become older, I have noticed that the response may be muted because an aqueous tear deficiency develops. Sjögren’s syndrome patients and patients with other auto-immune disorders also may show less benefit from androgen treatment due to their severe tear deficiency. Cyclosporine and punctal occlusion also is usually required in these patients.
I see an increasing number of men with “male menopause” experiencing DED. Often times they have already been diagnosed with low testosterone.
If this is suspected and they are not already on supplementation, I will order a testosterone level. I have seen men with levels less than 300 ng/dl who are often symptomatic with dry eyes and sometimes symptomatic with even less than 400 ng/dl.
I prescribe a higher 0.5% concentration testosterone eye drop for men. If they have systemic symptoms of androgen deficiency such as fatigue, night sweats, or erectile dysfunction, I will refer them to their primary-care physician for transdermal or injectable testosterone supplementation rather than prescribe an eye drop formulation.
From a medico-legal standpoint, it also is prudent for them to have a current prostate exam and a PAS level drawn.
I continue to note that current literature and conferences discussing DED fail to explore the androgen connection and this avenue of treatment despite the revelations of valid scientific research.
A commercial androgen eye drop is not yet available but does appear to be in a pharmaceutical pipeline.
For patients who get inadequate relief from the usual treatment and who fit the criteria suggested, I would encourage physicians to prescribe this aqueous soluble testosterone eye drop formulated by Leiter’s Pharmacy.
T.L. Dawson, MD