I had never heard of anyone ingesting “Neil Smith oil.” I had no idea what my patient was talking about, when she said that she bought “Neil Smith Oil” in Maryland–apparently, it is Cannabidiol oil (Reference 3), she took it as she was having chronic pain.
What happened next was very scary and she says she would never take it again. The room started spinning, she almost passed out and could barely make it to her bed before she passed out. She wasn’t even able to call 911 until after she woke up.
I do not know how many case reports there are on the safety of CBD oil, but for now, I would not recommend trying it.
Some patients have used CBD oil or marijuana as a way of decreasing eye pressure. This is also not recommended. A recent study by Miller et.al, showed that CBD can increase intraocular pressure (Reference 1) (particularly if you are a male rat). Also the American Academy of Ophthalmology does not recommend using Marijuana (Reference 2).
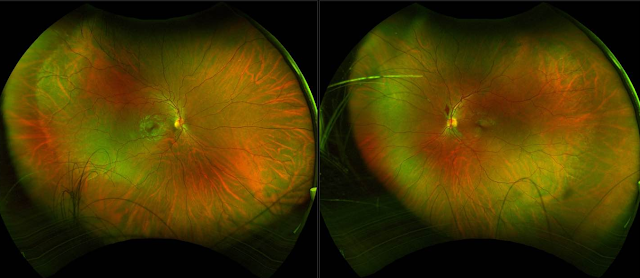
Recently I had a male patient come in with the following:
We still wonder if the new flame hemorrhage in the retina is from the marijuana or not.
He had no recent trauma, coughing, etc.
Unusual case of “excessive marijuana” (pt’s own words) use possibly leading to a flame hemorrhage in the left eye in a young 26yo male. He has very narrow angles ((ACV 76)) and discs at risk. Marijuana likely increased his eye pressure and led to this early sign of a vein occlusion. Has anyone seen this before? Blood work was negative per pt in recent past but repeating. No hx of DM, HTN, etc
SLC
PS: I did find this point interesting: that the diurnal variation of one’s pressure can be sex-dependent.
Our results also raise the possibility that the diurnal variation of IOP, in which GPR18 plays a role,35 is sex-dependent.
Reference 2
AAO Does not recommend Marijuana to treat Glaucoma.
Does Marijuana Help Treat Glaucoma or Other Eye Conditions?
Cannabis and currently available compounds derived from marijuana – like CBD – are not an adequate treatment for glaucoma, or any eye condition. To treat glaucoma, eye pressure must be managed 24 hours a day. Marijuana is not a practical treatment for constant use. And more research is still needed into the exact effects of cannabis and cannabis compounds on eye pressure and glaucoma.
The American Academy of Ophthalmology does not recommend marijuana or other cannabis products for the treatment of glaucoma. The American Glaucoma Society and the Canadian Ophthalmological Society agree.
Several current, effective treatments for glaucoma are more reliable and safer than marijuana. If you have glaucoma, you should follow your ophthalmologist’s advice to get the treatment that’s right for you.
The bottom line about marijuana and glaucoma is:
- The largest association of eye physicians and surgeons in the world does not endorse cannabis or its derivatives as a glaucoma treatment.
- Do not self-medicate with marijuana in an attempt to treat glaucoma. You can lose your vision if you don’t have a reliable, effective treatment for glaucoma.
- Speak with your ophthalmologist to find the glaucoma treatment option that’s best for you.
- Tell your doctor if you do use marijuana regularly.
What is the Connection Between Glaucoma and Marijuana?
Glaucoma is an eye condition in which the optic nerve becomes damaged over time, first reducing peripheral vision before possibly leading to total blindness. One cause of optic nerve damage in glaucoma is higher-than-normal eye pressure (intraocular pressure or IOP).
As marijuana has been legalized for medical or recreational use in more U.S. states and Canada, it has become more visible and discussed as a possible treatment for many health conditions. Research in the 1970s and 1980s did show a measurable decrease in intraocular pressure for three or four hours after smoking cannabis or ingesting THC as a pill or injection. But to treat glaucoma and save vision, eye pressure has to be controlled 24 hours a day.
To reduce intraocular pressure by 3 to 5 mm Hg — and maintain that reduction — you would have to ingest about 18 to 20 mg of THC six to eight times a day, every day. The possible negative effects on mood, mental clarity and (if smoked) lung health would be significant. You would not be able to drive, operate machinery or engage in many common activities. In addition, the cost of using marijuana every three to four hours, every day makes it cost-prohibitive for most patients.
As a comparison, alcohol also has a moderate intraocular pressure-lowering effect for an hour or so after a drink. But no doctor would recommend that you drink alcohol every hour to treat glaucoma. Many other effective treatments are available that don’t have the side-effects of alcohol.
Studies Haven’t Proven That THC is Effective or Reliable for Glaucoma Treatment
Studies have been done on THC eye drops, pills and cigarettes. Eye drops led to burning, irritated eyes and were shown to not lower eye pressure. A sublingual (placed in the mouth under the tongue) THC compound found no reduction in intraocular pressure. For another study, glaucoma patients were offered THC-containing pills and/or cigarettes. Within nine months all of them asked to stop due to side effects.
As scientists learn more about glaucoma, they have also come to understand that high intraocular pressure in the fluid at the front of the eye is not the only cause of optic nerve damage. Increasing evidence shows that reduced flow of blood to the optic nerve may also cause damage in patients with glaucoma. Marijuana not only lowers eye pressure, it also lowers blood pressure throughout the body. As a result, marijuana has the potential to lower the blood flow to the optic nerve, effectively canceling out the benefit of lowered intraocular pressure.
What About CBD for Glaucoma?
In recent years, CBD has received a lot of attention and scrutiny. CBD is a derivative of cannabis that doesn’t have mood-altering effects. But just like cannabis that’s smoked or eaten, there is no accepted, current research that shows CBD to be an effective treatment for glaucoma. In fact, one recent study showed that CBD may actually increase IOP, which would make glaucoma worse.
What is the Future of Marijuana for Glaucoma Treatment?
Currently, the only way to control glaucoma and prevent vision loss is to lower the pressure in your eye. Your ophthalmologist can treat glaucoma with medication, such as prescription eye drops, or surgery, depending on the type of glaucoma and how severe it is.
Scientists are exploring whether the active ingredients in marijuana may yet offer a glaucoma treatment. If the effects of cannabis compounds can be isolated, made to be long-acting, and the side effects eliminated, they may lead to new treatments in the future. However, such developments require more research and are years away from becoming a reality.
Ref 3
CBD oil being sold in Lincoln
By Ellis Wiltsey |
Posted: Fri 10:53 PM, Nov 16, 2018






Cannabidiol or CBD oil is a hot topic nationally as of late. It’s commonly used for pain relief and for the most part is illegal in Nebraska, but many including a former Husker great use it for medicinal purposes.
Neil Smith is most known for his work on the football field, at both Nebraska and then in the NFL. These days, he struggles with chronic pain, which he says CBD oil helps relieve almost instantly. The Attorney General and the Nebraska Legislature still say that CBD in that capacity, is illegal.
“Medical marijuana comes from the cannabis plant,” says Jamie Woolard of CBD American Shaman “our product comes from the hemp plant. The cannabis plant contains a high level of THC, which very simply is what makes people high”
CBD American Shaman recently opened its first store in Lincoln near 23rd Street and Cornhusker Highway. It carries over 125 different products that contain the oil. Neil Smith is a spokesperson for the brand and uses the products in his everyday life.
“I don’t have the aching headaches no more,” said Smith “I’ve started sleeping a little better at night. The main objective is that my pain level is really really gotten under control and I can say that I am pain free”
CBD in any capacity, other than products obtained and approved by a UNMC study or in a drug product approved by the FDA, remain illegal in Nebraska. The Attorney General re-issued a statement today of the law which said “Cannabidiol has been and continues to be included in Nebraska’s Uniform Controlled Substances Act’s legal definition of ‘marijuana'”
CBD American Shaman says it does not believe what they’re doing is illegal.
“I would say read the last line in the statement that was put out today,” said Woolard “apparently they charged someone with selling a CBD product and it says in the last line the court threw it out because they didn’t have enough evidence to prove it was a drug, this is not a drug”
The store also says they hope to open up the conversation about CBD oil.
“This is changing people’s lives,” said Smith “and when I say changing people’s lives in a positive way we are trying to bring awareness to people that want to bring awareness to people’s bodies”
The Attorney General told law enforcement that CBD should be treated as a schedule one controlled substance and act accordingly. As for CBD American Shaman, they say despite the message their products will remain on shelves.







