It appears True Tear does work and that it does likely stimulate the goblet cells and meibomian gland cells to produce. However, I have not seen any improvement in meibography with True Tear. Also there are some concerns on the quality of the studies and there are many patient who feel it did not help them.
Currently, True Tear is offered at most locations for around $700-1100. Most insurances do not reimburse for True Tear, so it is an expensive piece of dry eye equipment.

|
How Does True Tear Work And Why Does “Picking Your Nose” NOT work as well:
I am still not 100% sure I can answer the last part of this question since someone who really sticks a q tip, feather, rolled up tissue, or finger high up in the nose can induce stimulate the nerve that induces sneezing, tearing, and even salivation.
|
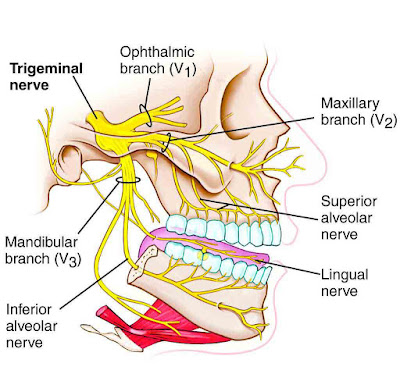
| The branches of the Trigeminal Nerve (or Cranial Nerve 5) extend to the mouth, nose, and eyes. |
What are the Positives of True Tear: First it does not involve the use of any preservatives or chemicals on the eye. Second, it usually increase tear production immediately. Third, there is evidence to show it increases meibomian gland lipid production. Fourth, it can be used as needed by the patient. Fifth, it seems to be very safe with no long term risks.
The Clinical Data
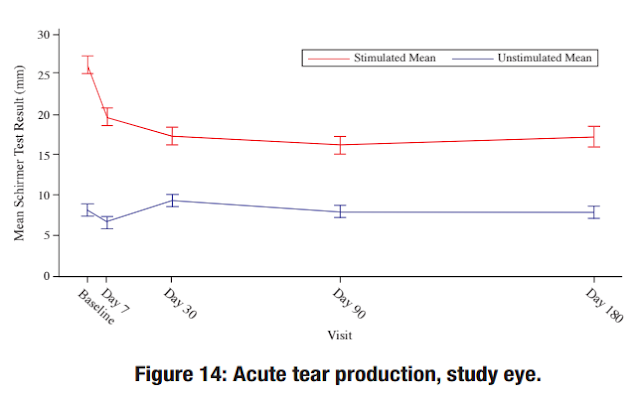
A second study of 97 adults evaluated aqueous deficient dry eye patients for 6 months comparing a treated eye to an untreated fellow eye in the same patients. The inclusion criteria for enrolling in this study was a baseline OSDI score of at least 23, a baseline Schirmer test with anesthetic of ≤ 10 mm in 5 minutes, and corneal fluorescein staining in the same eye. Patients were instructed to use the device at least twice a day for no longer than 3 minutes at a time, and no more than 10 times a day for the enrolled “study eye.” The fellow eye was not to be to stimulated. Patients were seen for follow-up on day 7, 30, 90, and 180.
The mean difference in Schirmer score (stimulated eye versus unstimulated) was
- 18.0 mm on day 0
- 13.1 mm on day 7
- 8.1 mm on day 30
- 8.3 mm on day 90
- 9.4 mm on day 180.
Based on these results it does appear that the initial tear production response to TrueTear is greater than it’s continued response with time, and with continued use the effectiveness settles to a stable level after about 1 month of use.
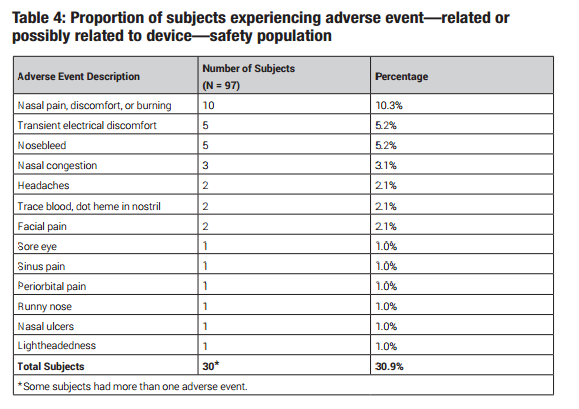
There were no serious adverse events reported. The most common side effects were “nasal discomfort” at 10.3%. Reps report that the device emits a “tingling” sensation, that could be strong enough to cause a sneeze when used on higher settings. Dr. White below noted excessive sneezing initially that subsided after frequent use. That is why starting on a lower level device setting is advisable and only turning the voltage up if no sensation occurs.
This device is NOT approved for
- patients with implanted neurostimulator devices like pacemakers or wearable defibrillators
- patients under 22 years old or pregnancy (it’s safety has not been studied at this time)
- use in water (a bath or shower), or around flammable gasses or shortwave devices
- patients with nasal or sinus surgery or untreated nasal infections
- patients with chronic or frequent nosebleeds, a bleeding disorder (eg, hemophilia), or another condition that can lead to increased bleeding
- patients with a known allergy to the hydrogel material the nasal tip is made from
How often would my patients use this?
The company recommends starting with twice daily use at Level 2 setting, with stimulation times of up to 3 minutes per use for patients with dry eye disease and aqueous deficiency. The maximum usage possible is 10 times a day (the device is limited to only 30 total minutes of neurostimulation per day). There are multiple voltage settings, levels 1 to 5, to provide increasing levels of stimulation. Allergan is reporting up to 4 hours of improved tear production per use.
|
Future Studies:
I must admit I have a crazy research idea: does True Tear help shorten the course of a cold or sinus infection? I have always suspected that people who make themselves sneeze early on in a cold, shorten the course of their cold. I wonder if True Tear’s use early on in a cold could shorten the cold’s duration because it makes one sneeze more….just an idea.
References:
This device is NOT approved for
- patients with implanted neurostimulator devices like pacemakers or wearable defibrillators
- patients under 22 years old or pregnancy (it’s safety has not been studied at this time)
- use in water (a bath or shower), or around flammable gasses or shortwave devices
- patients with nasal or sinus surgery or untreated nasal infections
- patients with chronic or frequent nosebleeds, a bleeding disorder (eg, hemophilia), or another condition that can lead to increased bleeding
- patients with a known allergy to the hydrogel material the nasal tip is made from
https://www.truetear.com/Content/pdfs/TrueTear-frequently-asked-questions.pdf
This is a very funny review of True Tear by an eye surgeon. But he is a consultant.
TrueTear: The latest buzz
– Jerry Seinfeld
The science behind TrueTear
How it will work
- For more information:
- Darrell E. White, MD, can be reached at SkyVision Centers, 2237 Crocker Road, Suite 100, Westlake, OH 44145; email: dwhite2@skyvisioncenters.com.
FREQUENTLY ASKED QUESTIONS
Frequently Asked Questions
Do I need a prescription to use the device?
Yes. You need a prescription from your eye care professional to obtain the TrueTear™ device.
How and where can I purchase the device?
The TrueTear™ device is a prescription-only device.
Ask your eye care professional about TrueTear™.
Is there a warranty available for the device?
Allergan warrants to the original purchaser of the TrueTear™ device that the device is free from defects
in materials and workmanship for three (3) years from the date of original purchase. This warranty
extends to only the original purchaser and is not transferable. Keep your invoice or receipt safe as this
is your proof of purchase, and the date marked on it shall be deemed the date of purchase. For all
warranty terms and conditions, please see the Instructions for Use that accompanied your TrueTear™
device or truetear.com.
USING THE DEVICE
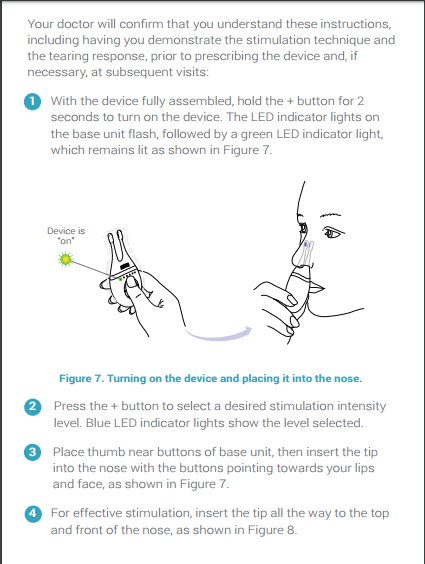
How far do I need to insert the tip into my nose?
Insert the tip into your nose as far as is comfortable. Make sure the tip is inserted all the way to the top
and front of the nose.
How do I know if the stimulation is working?
You are stimulating the correct location if you feel a tingling sensation in your nose and your eyes begin
to tear.
How soon will I feel my eyes tear?
Tearing can occur within seconds. Depending on your individual anatomy and tear deficiency, tearing
can take a shorter or longer time.
What stimulation level do I have to use to tear?
Stimulation intensity level is different for every patient and can vary on any given day. The TrueTear™
device allows you to adjust your intensity level according to your own needs. Most patients started at
level 2 in the clinical trial.
Please see Indication and Important Safety Information on page 6.
FREQUENTLY ASKED QUESTIONS
3
Does it matter if I stimulate for 30 seconds or 3 minutes?
Stimulation can vary depending on the individual patient. Stimulate until you see/feel tear production.
Stimulation longer than 3 minutes is not recommended, and the patient should wait for at least 60
minutes before stimulating again.
How often and how long do I need to use the device?
The device is capable of single-day stimulation up to a limit of 30 minutes. Stimulation longer than
3 minutes per session is not recommended, and the patient should wait for at least 60 minutes before
stimulating again. For most patients, using the device at least twice a day as needed is recommended.
Refer to the Instructions for Use or check with your eye doctor.
Can I use the device in the shower?
No. Do not use the TrueTear™ device in the bath or shower.
How long do I need to charge the device?
Charging time is dependent on how often you use the device. It normally takes 4 hours to complete
a full charge. We recommend charging the device overnight.
My device shuts off automatically after 1 minute. Is this normal?
Yes. The TrueTear™ device has a built-in timer allowing for 1 minute of stimulation each time. For longer
stimulation time, simply start another cycle, although stimulation longer than 3 minutes per session is
not recommended, and the patient should wait for at least 60 minutes before stimulating again.
SIDE EFFECTS
Will I experience nasal pain while using TrueTear™?
In a 180-day study, 10 out of 97 patients (10.3%) reported nasal pain, discomfort or burning. If you
experience pain, discomfort or burning while using TrueTear™, remove the device immediately and
report this adverse event to 1-800-678-1605 and talk to your eye doctor.
Is it possible to feel an electrical shock in my nose when the TrueTear™
tip is inserted?
In a 180-day study, 5 out of 97 patients (5.2%) reported temporary electrical discomfort (a mild shock).
If you feel a mild shock while using TrueTear™, remove the device immediately and report this adverse
event to 1-800-678-1605 and talk to your eye doctor.
Please see Indication and Important Safety Information on page 6.
FREQUENTLY ASKED QUESTIONS
Can using TrueTear™ cause nosebleeds?
In a 180-day study, 5 out of 97 patients (5.2%) reported a device-related nosebleed. If your nose begins
to bleed while using TrueTear™, remove the device immediately and report this adverse event to
1-800-678-1605 and talk to your doctor.
Should I be aware of any other side effects?
There were additional side effects reported during the clinical studies. For a complete list of additional
adverse events, please consult the
Instructions for Use.
DISPOSABLE TIPS
Can I use the tip for more than one day?
No. The tip should be replaced daily or every 24 hours. The tip contains a hydrogel that wears down
after one day of use. Once the hydrogel is dried out, the tip will no longer provide stimulation.
Additionally, make sure to use the tip before the expiration date printed on the packaging.
Can I clean the tip by running it under hot or cold water?
No. To clean the TrueTear™ device, you can use an alcohol pad to gently wipe the daily disposable
tip, being careful not to rub the hydrogel areas.
How do I clean my base unit, charger, and cover?
Use alcohol pads to clean the durable parts of the device including the base, charger, and cover.
Clean the inside of the cover weekly or more often as needed to ensure proper hygiene.
Are the tip and cover dishwasher safe?
No, they are not. The best way to clean them is by wiping them with alcohol pads.
Can I throw the tip away in a regular trash can?
Yes. The TrueTear™ daily diposable tip can go in the regular trash for disposal.
Please see Indication and Important Safety Information on page 6.
FREQUENTLY ASKED QUESTIONS:
MOBILE APP
What does the TrueTear™ mobile app do?
The app is designed to help you view and track your device usage and battery level, learn more ways
for using the device, and get assistance if you encounter any problems with the device. Since
environmental conditions such as humidity, temperature, pollen count, and pollution index may affect
your eyes, these and other local weather information are shown throughout the app.
Do I have to use the TrueTear™ mobile app in order to use my TrueTear™ device?
No. The TrueTear™ mobile app is not required in order to use your TrueTear™ device.
How much does the TrueTear™ mobile app cost, and does it work on both iOS™
and Android®?
The TrueTear™ mobile app is a free app that is currently available for iOS only. We are working
on one for Android.
How do I pair and sync my TrueTear™ device and App?
Make sure Bluetooth® is enabled on your mobile device. Once you have created an account, the app
will walk you through the pairing process. Enter the serial number located on the back of the base unit,
into the app. Hold down the “-” button on the base unit for 3 seconds until a blue LED indicator flashes,
then tap the “Pair Now” button in the app.
To sync after use: with your mobile device nearby, simply use the TrueTear™ device as usual or place
the base unit into the charger. During these times the TrueTear™ device will automatically connect with
your paired mobile device.
Can I pair my TrueTear™ device with more than one mobile device?
Yes. You can pair your TrueTear™ device with multiple mobile devices; however, your device can only
be actively paired with one mobile device at any given time.
Why are all of the icons greyed out?
To protect your device data, most of the features within the app such as pairing a device require you to
have an account and an active internet connection. Make sure you’re signed in to your account and are
either connected to a Wi-Fi network or have good cell service.
What do the trend charts do?
Trend charts in the TrueTear™ mobile app summarize your use over the last two weeks by time of day
and day of the week. You can use these charts yourself or share them with your healthcare provider to
get feedback on your prescribed usage.
Please see Indication and Important Safety Information on page 6.
FREQUENTLY ASKED QUESTIONS
6 TRU105777_V3 08/ 1 7
TRUETEAR™ CONSUMER INDICATION AND
IMPORTANT SAFETY INFORMATION
INDICATION
TrueTear™ provides a temporary increase in tear production during neurostimulation in adult patients.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Do not use TrueTear™ if you have a cardiac pacemaker, implanted or wearable defibrillator, or other
implanted metallic or electronic device (eg, cochlear implant) in the head or neck, chronic or recurrent
nosebleeds, a bleeding disorder (eg, hemophilia), a condition that can lead to increased bleeding, or a
known hypersensitivity (allergy) to the hydrogel material.
WARNINGS
Do not use TrueTear™ around electronic monitoring equipment (eg, heart monitors or electrocardiogram
alarms), in the bath/shower, while driving, operating machinery, or during activity in which sneezing/
watery eyes may cause risk, areas other than the nose, within 3 feet of shortwave or microwave therapy
equipment, around flammable anesthetic mixture (air, oxygen or nitrous oxide). Do not continue using
TrueTear™ if your nose is irritated. Safety/effectiveness not established for longer than 6 months or for
treating aqueous-deficient dry eye disease. Safety not established in pregnancy, patients under 22 years
of age, patients with nasal or sinus surgery (including nasal cautery) or significant trauma, severe nasal
airway obstruction or vascularized polyp; active, severe systemic or chronic seasonal allergies; rhinitis or
sinusitis requiring treatment; untreated nasal infection; and disabling arthritis, neuropathy, severe
dexterity impairment or limited motor coordination.
PRECAUTIONS
Consult your doctor on TrueTear™ instructions before use and on discontinuing use if pain, discomfort or
numbness in the nose persists after reducing for higher levels/longer sessions. Remove studs, nose
rings, or other nose jewelry before use. Do not use prescription eye medications or nasal sprays 30
minutes before or after using TrueTear™. Consult your doctor before use if you have suspected or
diagnosed heart disease. Keep away from children.
ADVERSE EVENTS
Nasal pain, discomfort or burning; short-term electrical discomfort; nosebleed; nasal congestion;
headaches; trace blood in nostril; facial pain; sore eye; sinus pain; pain around the eye; runny nose;
nasal ulcers; and light-headedness.
Caution: Federal law restricts this device to sale by or on the order of a licensed physician. For the full
Directions for Use, please visit www.allergan.com/truetear/usa.htm or call 1-800-678- 1605. Please call
1-800-433-8871 to report an adverse event.
© 2017 ALLER GAN . ALL R I G H T S R ESERV ED. T RUE T E AR™ AND I T S D ESIGN AR E T R ADEMAR KS OF
OCULE V E, IN C . , AN ALLER G AN AFFILI A T E .