What is the BEST Source of Stem Cells?
I am beginning a research protocol to inject Stem Cells into the Meibomian Gland in patients who have not been helped by other treatments.
Initially I planned to use a patient’s own Autologous Serum which we have been making for years at Harvard and now at Visionary Eye Doctors in Washington DC area. But it looks like there are not enough stem cells in Autologous Serum and a better source would be adipose (fat tissue).
Mesenchymal Stem Cells (MSC) are multipotent cells, which means they can turn into or differentiate into a variety of cell types, including: osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells) and adipocytes (fat cells). These stem cells have the potential to not only turn into the cell type of where it is injected but also to heal damaged cells in its area.
MSCs can be induced to differentiate into osteocytes, chondrocytes and adipocytes under certain conditions and also possess immunomodulatory function with low immunogenicity, which makes MSCs a promising choice in regenerative medicine and cellular therapy
The problem is that there is little head to head research on where is the best place to obtain stem cells, such as from one’s bone marrow versus your fat/adipose tissue versus your autologous serum. We know embryonic stem cells have proved thus far to be a failure in helping rejuvenate cells and organs in almost all cases. Still many products are on the market which say they will rejuvenate your stem cells. There are many good reviews on many products, such as this one, below, but there are no randomized, controlled studies to say they help compared to controls.

We have yet to prove that Stem Cells can turn into Meibomian Gland Cells, but that is what I hope to investigate.
I would add in Meibomian Gland Death/Disease on the below photo.
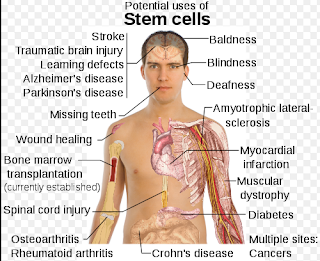
So the question is now: What is the Best Source of Stem Cells to try to inject into the Meibomian Gland.
This is a slightly controversial issue. Which is better, stem cells from Fat (adipose means fat) or from Bone Marrow?
Apparently, there is a higher concentration of stem cells in Fat compared to Bone Marrow. Further, it appears that stems cells from Fat behave a little differently from stem cells from Bone Marrow.
It is not clear if a mixture of Stem Cells Derived from Fat and Bone-Marrow together is better than one alone. For now, it is easier to extract Fat from a person than Bone Marrow.
One study below suggests the following: the disease you are trying to treat may dictate where you should get the stem cells from (Fat, Bone Marrow, or Umbilical Cord or Autologous Serum: 10% AS may be a contender: see below).
I believe for the Meibomian Gland, Fat/Adipose tissue is the best to use first. Eventually, I hope to try Stem Cells from Fat versus Bone Marrow vs Autologous Serum.
Our first protocol will be using Fat/Adipose tissue, which is safe to extract and is not as painful as getting Bone Marrow aspirates for most patients.
Sandra Lora Cremers, MD, FACS
Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use.
Abstract
OBJECTIVE:
Mesenchymal stem cells (MSC) are promising candidates for cell-based therapies. One major obstacle for their clinical use is the biosafety of fetal calf serum (FCS), which is a crucial part of all media currently used for the culture of MSC.
METHODS:
Nine donors each contributed 5 mL of bone marrow aspirate. Isolation of MSC was conducted according to Caplan et al., although for expansion we used low-density seeding with 20 MSC/cm2. Four different media A, B, C, and D were tested, containing 1%, 3%, or 10% autologous serum (AS), or 10% selected FCS, respectively. MSC were cultured on 24-well plates until passage 2 and counted under the microscope at regular intervals. Osteogenic and adipogenic differentiation were induced in vitro by using a modified standard cocktail and were evaluated semi-quantitatively through a microscope.
RESULTS:
Isolation of MSC after 3 days appeared best in media C with almost always C>D congruent with B>A. Proliferation was exponential with generally C>D>B>A. Morphologically, MSC isolated and expanded in medium C were indistinguishable from those in medium D. Phenotypic markers of MSC grown in medium C were: CD34-, CD45-, CD90+, CD105+, MHC class I+, MHC class II-, similar to MSC isolated and grown in medium D. Moreover, MSC grown in medium C showed more osteogenic potential than those from medium D in all cases: C+++, D++, B+, A 0. Cells retained their immaturity as shown by adipogenic differentiation and it always was: D+++, C++, B+, A 0.
CONCLUSIONS:
Growth of MSC in a FCS-free medium is feasible without addition of growth factors. Ten percent AS appears at least as good as 10% FCS with regard to both isolation and expansion of human MSC, while 1% and 3% AS appear inferior. With respect to osteogenic differentiation, 10% AS proved superior to the other serum conditions.
Sci Rep. 2017 Apr 25;7(1):1160. doi: 10.1038/s41598-017-01294-2.
miR-301b~miR-130b-PPARγ axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins.
Abstract
Mesenchymal stem cells (MSCs) have been widely used in regenerative medicine and cellular therapy due to their multi-lineage differentiation potential and immunomodulatory function. The applicability of MSCs also depends on their cellular sources and in vivo functions. Here in this study, we systematically compared the morphologic characteristics, immunophenotypes and the adipogenic differentiation of MSCs derived from umbilical cord (UC), adipose tissue (Ad) and bone marrow (BM). We found that the three tissues-derived MSCs displayed decreased adipogenic capacity in the order: Ad-MSC > BM-MSC > UC-MSC, and no morphologic and immunophenotypic differences were observed. Mechanistic investigation revealed a miR-301b~miR-130b-PPARγ axis, whose expression pattern in UC-MSC, Ad-MSC and BM-MSC significantly correlates with their adipogenic capacity. Our results come up with a potential mechanism to elucidate the differential adipogenesis of Ad-MSC, BM-MSC and UC-MSC, which would provide instructional advice for which source of MSCs to choose according to a certain clinical purpose. Furthermore, the miR-301b~miR-130b-PPARγ axis may also be used as a potential therapeutic target for the disorders associated with MSCs-mediated abnormal adipogenesis.
Sandra Lora Cremers, MD, FACS
Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9.
Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.
Abstract
Mesenchymal stem/stromal cells (MSCs) comprise a heterogeneous population of cells with multilineage differentiation potential, the ability to modulate oxidative stress, and secrete various cytokines and growth factors that can have immunomodulatory, angiogenic, anti-inflammatory and anti-apoptotic effects. Recent data indicate that these paracrine factors may play a key role in MSC-mediated effects in modulating various acute and chronic pathological conditions. MSCs are found in virtually all organs of the body. Bone marrow-derived MSCs (BM-MSCs) were discovered first, and the bone marrow was considered the main source of MSCs for clinical application. Subsequently, MSCs have been isolated from various other sources with the adipose tissue, serving as one of the alternatives to bone marrow. Adipose tissue-derived MSCs (ASCs) can be more easily isolated; this approach is safer, and also, considerably larger amounts of ASCs can be obtained compared with the bone marrow. ASCs and BM-MSCs share many biological characteristics; however, there are some differences in their immunophenotype, differentiation potential, transcriptome, proteome, and immunomodulatory activity. Some of these differences may represent specific features of BM-MSCs and ASCs, while others are suggestive of the inherent heterogeneity of both BM-MSC and ASC populations. Still other differences may simply be related to different isolation and culture protocols. Most importantly, despite the minor differences between these MSC populations, ASCs seem to be as effective as BM-MSCs in clinical application, and, in some cases, may be better suited than BM-MSCs. In this review, we will examine in detail the ontology, biology, preclinical, and clinical application of BM-MSCs versus ASCs.
Differential inflammatory networks distinguish responses to bone marrow-derived vs. adipose-derived mesenchymal stem cell therapies in vascularized composite allotransplantation.
Abstract
BACKGROUND:
Vascularized composite allotransplantation (VCA) is aimed at enabling injured individuals to return to their previous lifestyles. Unfortunately, VCA induces an immune/inflammatory response, which mandates lifelong, systemic immunosuppression, with attendant detrimental effects. Mesenchymal stem cells (MSC) – both adipose-derived (AD-MSC) and bone marrow-derived (BM-MSC) – can reprogram inflammation and have been suggested as an alternative to immunosuppression, but their mechanism of action is as yet not fully elucidated. We sought to gain insights into these mechanisms using a systems biology approach.
METHODS:
PKH26 (red) dye-labeled AD-MSC or BM-MSC were administered intravenously to Lewis rat recipients of mismatched Brown Norway hindlimb transplants. Short course tacrolimus (FK-506) monotherapy was withdrawn at POD 21. Sera were collected at 4, 6, 18 weeks, assayed for 29 inflammatory/immune mediators, and the resultant data were analyzed using Dynamic Network Analysis (DyNA), Dynamic Bayesian Network (DyBN) inference, and Principal Component Analysis (PCA).
RESULTS:
DyNA network complexity decreased with time in AD-MSC rats, but increased in BM-MSC rats. DyBN and PCA suggested mostly different central nodes and principal characteristics, respectively, in AD-MSC vs. BM-MSC rats.
CONCLUSIONS:
AD-MSC and BM-MSC are associated with both overlapping and distinct dynamic networks and principal characteristics of inflammatory/immune mediators in VCA grafts with short course tacrolimus induction therapy. The decreasing inflammatory complexity of dynamic networks in the presence of AD-MSC supports the previously suggested role for regulatory T cells induced by AD-MSC. The finding of some overlapping and some distinct central nodes and principal characteristics suggests the role of key mediators in the response to VCA in general, as well as potentially differential roles for other mediators ascribed to the actions of the different MSC populations. Thus, combined in vivo/in silico strategies may yield novel means of optimizing MSC therapy for VCA
—-
Again it is controversial:
This study below done on rats and not humans seems to indicate that Stem Cells from Bone Marrow may be better if you are dealing with a disease process involving bone:
Potential of Osteoblastic Cells Derived from Bone Marrow and Adipose Tissue Associated with a Polymer/Ceramic Composite to Repair Bone Tissue.
Freitas GP1, Lopes HB1, Almeida ALG1, Abuna RPF1, Gimenes R2, Souza LEB3,4, Covas DT3,4, Beloti MM1, Rosa AL5.
Abstract
One of the tissue engineering strategies to promote bone regeneration is the association of cells and biomaterials. In this context, the aim of this study was to evaluate if cell source, either from bone marrow or adipose tissue, affects bone repair induced by osteoblastic cells associated with a membrane of poly(vinylidene-trifluoroethylene)/barium titanate (PVDF-TrFE/BT). Mesenchymal stem cells (MSC) were isolated from rat bone marrow and adipose tissue and characterized by detection of several surface markers. Also, both cell populations were cultured under osteogenic conditions and it was observed that MSC from bone marrow were more osteogenic than MSC from adipose tissue. The bone repair was evaluated in rat calvarial defects implanted with PVDF-TrFE/BT membrane and locally injected with (1) osteoblastic cells differentiated from MSC from bone marrow, (2) osteoblastic cells differentiated from MSC from adipose tissue or (3) phosphate-buffered saline. Luciferase-expressing osteoblastic cells derived from bone marrow and adipose tissue were detected in bone defects after cell injection during 25 days without difference in luciferin signal between cells from both sources. Corroborating the in vitro findings, osteoblastic cells from bone marrow combined with the PVDF-TrFE/BT membrane increased the bone formation, whereas osteoblastic cells from adipose tissue did not enhance the bone repair induced by the membrane itself. Based on these findings, it is possible to conclude that, by combining a membrane with cells in this rat model, cell source matters and that bone marrow could be a more suitable source of cells for therapies to engineer bone.



