The XEN GEL Implant (Allergan Inc., CA, USA) is an FDA approved implant that is inserted into the eye to connect the inner eye fluid with the outside area under the conjunctival. It has a 6-mm hydrophilic tube of collagen-derived gelatin cross-linked with glutaraldehyde. It has with a 45micron internal diameter and an external diameter that expands when wet to about 110microns. It is noninflammatory and reported to cause minimal extraocular fibrotic or vascular response to the implant material, though private reports of surrounding inflammation have been reported: even a colleague noted a “sock of inflammation” around one of the XEN implants.
It decreases eye pressure by creating a permanent drainage shunt from the AC to the subconjunctival space through a the XEN channel. This collagen device hydrates on contact with water within 1–2 min, bending and conforming to tissue. Rare reports of migration, imbedding in the iris (improved with a Yag laser), erosion through the conjunctiva,bending, curling, and corneal endothelial damage have been noticed but are rare.
XEN Gel implants may help prevent trabeculectomy surgery which is well known to have significant complications, such as endophthalmitis (I have seen more than 30 in my career), choroidal effusions (again have seen many such complications).
The XEN has been in the US for about 2.5 years. There is only 1 reported case of endophthalmitis in a patient who had diabetes. The majority of patients have done very well and have been able to get off their medication.
The indications are refractory glaucoma which means:
1. Failed maximum tolerated medications which for some patients may be 1-2 drops
2. HVF-Visual field progression
3. Non-compliance in such patients is also an indication.
The paradigm shift for Glaucoma treatment is thus now:
1. Drops, can add up to 3 types of drops but research shows the effectiveness of the addition as well as compliance decreases as we add more drops.
2. SLT or ALT, Laser Trabeculoplasty: this is generally very safe but may not be enough for some of our patients.
3. If a patient is having cataract surgery and has glaucoma, a patient should have
a. Goniotomy, Kahook: a common procedure where a sharp blade penetrates the Trabecular Meshwork or the drain of the eye to allow fluid to more easily exit the eye.

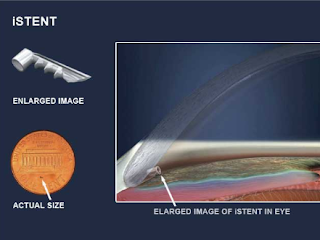
b. iStent: a titanium stent that keeps the drain of the eye open. The iStent Inject 0.23 x 0.36 mm creates two patent bypasses through the trabecular meshwork, resulting in a multi-directional flow through Schlemm’s canal.
4. XEN should be considered before trabeculectomy if a patient has refractory Glaucoma or has failed Goniotomy and/or iStent. It can be done with our WITHOUT cataract surgery.
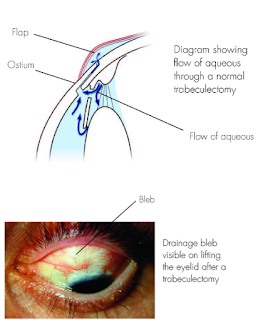
5. Trabeculectomy: is a surgical operation which lowers the eye pressure (ie, intraocular pressure (IOP) in patients with glaucoma. A small hole in the eye wall (sclera) is made with a metal punch hole machine and covered by a thin trap-door in the sclera. The fluid inside the eye known as aqueous humour, drains through the trap-door to a small reservoir or bleb just under the eye surface, hidden by the eyelid. The scleral flap’s trap-door is sutured (stitched) in a way that prevents aqueous humour from draining too quickly. By draining aqueous humour trabeculectomy reduces the pressure on the optic nerve and prevents or slows further damage and further loss of vision in glaucoma. Control of the eye pressure with a trabeculectomy or any procedure will not restore vision already lost from glaucoma.
Clearly, most patients and MDs prefer the least invasive option possible.
References:
https://eyewiki.aao.org/Xen_Glaucoma_Treatment_System
Xen Glaucoma Treatment System
Contents
[hide]
Background
Xen Gel Implant
Suitable and Non-suitable Patients
- Patients with uncontrolled glaucoma, primary open-angle glaucoma, and pseudoexfoliative or pigmentary glaucoma with open angles that are unresponsive to maximum tolerated medical therapy.[7][13]
The XEN gel stent is generally contraindicated under the following circumstances or conditions:
- Patients with angle ¬closure glaucoma where the angle has not been surgically opened; previous glaucoma shunt/valve or conjunctival scarring/pathologies in the target quadrant; active iris neovascularization or neovascularization of the iris within 6 months of the surgical date; active inflammation (eg, blepharitis, conjunctivitis, keratitis, and uveitis)[15]; anterior chamber intraocular lens; intraocular silicone oil; vitreous in the anterior chamber; impaired episcleral venous drainage (eg, Sturge–Weber or nanophthalmos or other evidence of elevated venous pressure) and known or suspected allergy or sensitivity to drugs required for the surgical procedure or any of the device components (eg, porcine products or glutaraldehyde).[15]
Surgical Procedure
- After topical anesthesia, 0.05–0.2 ml MMC (0.1–0.2 mg/ml) is injected with a 30-gauge needle in the superonasal quadrant and massaged over the area of anticipated XEN Gel Implant insertion (off-label use) or a conjunctival flap is made and MMC applied on sponges.[3] This induces a hydroexpansion, which reduces tissue resistance, preparing the space for the implant and supporting the bleb formation.[15]
- Cataract surgery, if planned, may be performed after this step using miotic drugs after intraocular lens (IOL) implantation and ophthalmic viscosurgical device (OVD) removal.[15]
- The intended area of placement in the supero-nasal quadrant, which is 3 mm from the limbus, is marked.[15]
- Small, self-sealing corneal incisions are made, and viscoelastic is used to fill the AC.
- Injector tip is placed through an inferotemporal clear corneal incision (~2mm), whereas a second instrument is utilized for counter-traction through a superotemporal paracentesis (1 mm)
- The inserter needle (double-beveled 27 gauge) is directed[15] through the main incision and across the AC toward the superonasal quadrant (mirrored gonioscope can be used to confirm placement, but it is not mandatory, it is used at the discretion of the surgeon)
- XEN Gel Implant should be placed anterior to Schlemm’s canal to avoid bleeding (under direct observation using a gonioscope)
- The sharp tip is engaged at or slightly anterior to the trabecular meshwork and advanced through the sclera[11]
- The needle is tunneled through the sclera coming out subconjunctivally 3.0 mm from the limbus, as previously marked, using a second instrument to provide countertraction via the side port
- A sliding mechanism is then pushed forward to initially deploy the stent and then to retract the needle into the hub[11], without drawing the implant back[15]
- The injector is then withdrawn, subconjunctival and AC XEN Gel Implant placement is confirmed. The ideal stent placement should leave 2.0 mm of exposed implant in the subconjunctival space (preferentially in a more supercial layer than the sub-Tenon space), 1.0 mm in the AC, and 3.0 mm tunneled through sclera, that provides resistance
- Viscoelastic is removed from the AC
- Bleb morphology and function may be obtained by forced infusion of fluid through paracentesis at the end of the procedure.[15]
Perioperative Management
- Patients are instructed to stop all glaucoma medications on the day of the operation.
- Patients are seen postoperative day 1 and followed up at the physician discretion.
- Postoperative topical regimen includes topical antibiotic prophylaxis for 4 weeks and topical corticosteroid 4 times each day for a month followed by a slow taper over the second month.[18][19]
- XEN implant location and subconjunctival bleb morphology require close biomicroscopic follow-up, complemented with UBM (FIG1) or AS-OCT.
- Needling may be performed in case of IOP increases and is greater than target IOP; there is a flat bleb or fibrotic bleb or the patient is at high risk of bleb failure.
Xen Implant Advantages
- Soft and flexible when wet;
- Minimally invasive, pre-loaded injector;
- Shorter surgical and recovery times;
- Less side effects: Eg. Prevents chronic hypotony by an intrinsic flow-limiting design[11]
- Keep postoperative options open, allowing physicians to use other IOP-reduction techniques that could be required after surgery [16]
Complications
Intra-operative complications:
- Misplacement of the XEN Gel Implant at the first attempt;
- Posterior placement of the implant, especially through ciliary body can lead to bleeding and hypotony;
- Subconjunctival or AC bleeding during the implantation.
Post-operative complications:
- Complications of relocation or reimplantation (misplaced XEN Fig);
- Wound leak;[13]
- Transient hypotony, AC shallowing, and choroidal detachment (less frequent with XEN 45);
- Internal ostium occlusion with a blood clot leading to raised IOP and flat bleb.
Late complications:
- Device erosion and exposure of implant (add XEN erosion Fig);
- Implant migration [13]: Dislocation into AC, XEN-iris touch;
- Bleb leak or dehiscence (may be due to thin or ischemic bleb with overfiltration);
- Reported cases of blebitis and persistent hypotony[22], suprachoroidal bleeding[23], conjunctival perforation and late seidel.[24]
Efficacy and Safety Profile
XEN Gel Implant Vs. Trabeculectomy
XEN Implant in Refractory Glaucoma
XEN-augmented Baerveldt surgical technique
Conclusion
References
- ↑ Quigley HA, Broman AT. The number of people with glaucoma world wide in 2010 and 2020. Br J Ophthalmol 2006; 90:262 – 267
- ↑ Tham Y C, Li X, Wong T Y, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and metaanalysis. Ophthalmology 2014; 121:2081 – 2090.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Ankita Chaudhary, Lauriane Salinas, Jacopo Guidotti, André Mermoud & Kaweh Mansouri (2017): XEN Gel Implant: a new surgical approach in glaucoma, Expert Review of Medical Devices, DOI: 10.1080/17434440.2018.1419060
- ↑ Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267.
- ↑ Boland MV, Ervin AM, Friedman DS, et al. Comparative effective- ness of treatments for open-angle glaucoma: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:271–279.
- ↑ Kass M , Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713.
- ↑ 7.0 7.1 Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol. 2002;120:1268–1279.
- ↑ Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953.
- ↑ American Academy of Ophthalmology Preferred Practice Patterns Committee GP. Ophthalmology. Chicago (IL): American Academy of Ophthalmology; 2010. Preferred practice pattern: primary open-angle glaucoma.
- ↑ Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301–1306.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 Green, A. Lind, J. Sheybani, A. Review of the Xen Gel Stent and Inn Focus MicroShunt. Curr Opin Ophthalmology. 2018, 28:000 – 000.
- ↑ Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140:16–22.
- ↑ 13.0 13.1 13.2 13.3 Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow- up. Am J Ophthalmol. 2012;153:789–803.
- ↑ Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 15.12 15.13 De Gregorio A, Pedrotti E, Stevan G, Bertoncello A, Morselli S. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice. Clinical Ophthalmology (Auckland, NZ). 2018;12:773-782. doi:10.2147/OPTH.S146919.
- ↑ 16.0 16.1 16.2 FDA approves Xen gel stent for glaucoma – American Academy of Ophthalmology. https://www.aao.org/headline/fda-approves-xen-gel-stent-glaucoma. Accessed May 20, 2018
- ↑ Sheybani A, Reitsamer H, Ahmed IIK. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:4789–4795.
- ↑ Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsi cation combined with a new ab interno gel stent to treat open-angle glaucoma: Pilot study. J Cataract Refract Surg. 2015;41(9): 1905–1909.
- ↑ Sheybani A, Dick B, Ahmed II. Early clinical results of a novel Ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–e696.
- ↑ 20.0 20.1 Antonio Maria Fea, Roberta Spinetta, Paola Maria Loredana Cannizzo, et al., “Evaluation of Bleb Morphology and Reduction in IOP and Glaucoma edication following Implantation of a Novel Gel Stent,” Journal of Ophthalmology, vol. 2017, Article ID 9364910, 9 pages, 2017. https://doi.org/10.1155/2017/9364910.
- ↑ Kerr, N. et al. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma, Clin Exp Ophthalmol. 2017 May;45(4):393-400. doi: 10.1111/ceo.12888.
- ↑ Sng CC, Wang J, Hau S, et al. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2017. doi: 10.1111/ceo.13087.
- ↑ Prokosch WV, Vossmerbaeumer U, Hoffmann E, et al. Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017;26:e261–63.
- ↑ Olate-Pérez A, Perez-Torregrosa VT, Gargallo-Benedicto A, et al. Management of conjunctival perforation and late Seidel after XEN surgery. Arch Soc Esp Oftalmol. 2017. doi: 10.1016/j. oftal.2017.10.002.
- ↑ Galal A, Bilgic A, Eltanamly R, et al. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246.
- ↑ De Gregoria A, Pedrotti E, Russo L, et al. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2017. doi: 10.1007/s/0792- 017-0571-x
- ↑ Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124:1579–1588.
- ↑ Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36.
- ↑ Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011;118:2180–2189.
- ↑ Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118:443–452.
- ↑ 31.0 31.1 D’Alessandro E, Guidotti JM, Mansouri K, et al. XEN-augmented Baerveldt: a new surgical technique for refractory glaucoma. J Glaucoma. 2017;26:e90–2.
- ↑ Sng CCA, Wang J, Barton K. Caution in using the XEN-augmented Baerveldt surgical technique. J Glaucoma. 2017;26:e257.
- ↑ Hohberger B, Welge-Lüen VC, Lämmer R. ICE-syndrome: a case report of implantation of a microbypass XEN gel stent after DMEK transplantation. J Glaucoma. 2017;26:e103–4.