The molecular cause of pain is very complex. Chronic pain is also not fully understood. It is clear though that chronic pain can lead to misery and affect all aspects of one’s life and family. Doctors and surgeons have many wonderful options to treat pain, but once in a while we see a patient that we have a tough time helping. This is very distressing for everyone!
Pietro, et. al., and Alshelh, et. al, have a great reviews below of how chronic pain develops.
The key cause is that when one’s nerves or body is hit with an insult (could be a previous virus/bacteria, genetics, trauma, surgery, aging, smoking, chemical, poor diet (?)), in some patients, the brain’s thalamus begins to fire out signals that result in the constant perception of pain.
Specifically:
1. Altered thalamic burst firing and thalamocortical dysrhythmia increased infra-slow oscillatory
activity throughout the ascending pain pathway, including within the spinal trigeminal nucleus, somatosensory thalamus, thalamic
reticular nucleus, and primary somatosensory cortex.
2. The spinal trigeminal
nucleus region displays increased regional homogeneity, consistent with a local spread of neural activity by astrocyte activation
3. In Healthy Patients: there is no increase in oscillatory behavior within the ascending pain pathway occurred during acute noxious stimuli. These data reveal increased oscillatory activity within the ascending pain pathway explains increased thalamocortical
oscillatory activity, a self-sustaining thalamocortical dysrhythmia, and the constant perception of pain.
4. After nerve injury, increased activity within primary afferent neurons results in excessive neurotransmitter release, likely glutamatergic, leading to more neural death, which likely involves local interneurons.
SLC
The relationship between thalamic GABA content
and resting cortical rhythm in neuropathic pain
Flavia Di Pietro1 | Paul M. Macey2 | Caroline D. Rae3 | Zeynab Alshelh1 |
Vaughan G. Macefield3,4 | E. Russell Vickers1 | Luke A. Henderson1
thought that the inhibitory action of the thalamic reticular nucleus is critical in setting these
rhythms. Our work and others’ has suggested that chronic pain that develops following nerve
injury, that is, neuropathic pain, results from altered thalamocortical rhythm, although whether this
dysrhythmia is associated with thalamic inhibitory function remains unknown. In this investigation,
we used electroencephalography and magnetic resonance spectroscopy to investigate cortical
power and thalamic GABAergic concentration in 20 patients with neuropathic pain and 20
pain-free controls. First, we found thalamocortical dysrhythmia in chronic orofacial neuropathic
pain; patients displayed greater power than controls over the 4–25 Hz frequency range, most
marked in the theta and low alpha bands. Furthermore, sensorimotor cortex displayed a strong
positive correlation between cortical power and pain intensity. Interestingly, we found no difference
in thalamic GABA concentration between pain subjects and control subjects. However, we
demonstrated significant linear relationships between thalamic GABA concentration and enhanced
cortical power in pain subjects but not controls. Whilst the difference in relationship between
thalamic GABA concentration and resting brain rhythm between chronic pain and control subjects
does not prove a cause and effect link, it is consistent with a role for thalamic inhibitory neurotransmitter
release, possibly from the thalamic reticular nucleus, in altered brain rhythms in
individuals with chronic neuropathic pain.
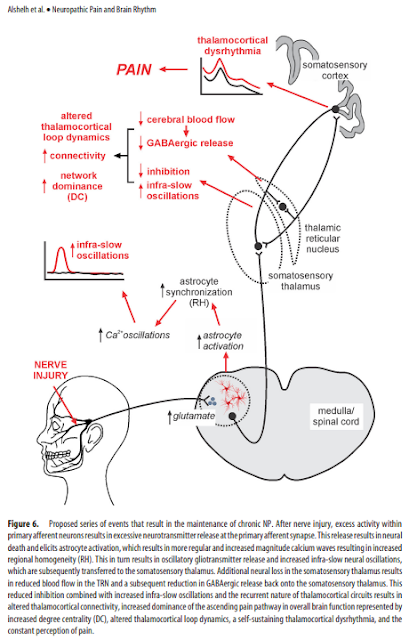
after nerve injury, increased activity within
primary afferent neurons results in excessive neurotransmitter
release, likely glutamatergic. This results in neural death, which
likely involves local interneurons because of the following: (1) in
animal models of NP, GABAergic interneuron loss occurs in the
dorsal horn, which is closely related to glutamate receptor activation
(de Novellis et al., 2004; Scholz et al., 2005); and (2) orofacial
NP is associated with reduced gray matter volume and mean
diffusivity within SpV (Wilcox et al., 2015). Excess neural discharge
also elicits astrocyte activation, spreading oscillating calcium
waves, oscillatory gliotransmitter release characterized by
increased regional homogeneity, and increased infra-slow neural
oscillations throughout the ascending pain pathway. Additionally,
local interneuron loss within the somatosensory thalamus
resulting from a similar mechanism to that which occurs in SpV
results in reduced TRN activity, reduced GABAergic output onto
the somatosensory thalamus (Henderson et al., 2013) and, in
combination with increased infra-slow oscillatory activity, results
in altered thalamocortical connectivity, increased dominance of
the ascending pain pathway characterized by increased degree
centrality, a self-sustaining thalamocortical dysrhythmia, and the
constant perception of pain (Fig. 6).
Although this model points to a critical role for the primary
afferent synapse in maintaining NP but does not exclude the
possibility that, in different individuals and in different NP conditions,
other sites along the ascending pain pathway play a role
in pain maintenance. Furthermore, it is possible that increased
infra-slow oscillations in regions other than the SpV, thalamus,
and S1 are involved in the maintenance of NP. For example, we
found altered infra-slow oscillatory power, degree centrality,
and/or regional homogeneity in the cerebellar, dorsolateral prefrontal,
mid-cingulate, and insular cortices in NP subjects. In
animal models of NP, astrocyte activation occurs in the cingulate
cortex, and a recent study suggests that this activation may be
related to sleep disturbances (Kuzumaki et al., 2007; Yamashita et
al., 2014). Furthermore, increased gene expression indicative of
astrocyte activation occurs in the prefrontal cortex of mice with
NP that is likely related to pain comorbidities, such as mood
disorders (Alvarado et al., 2013). Although the focus of this investigation
was on the ascending somatosensory pathway, it is
likely that changes in other brain regions, such as association
cortices, are involved in maintaining the emotional and cognitive
aspects of NP. It is also possible that the changes in infra-slow
oscillatory power reported here do not result from astrocyte–
neural interactions and may result simply from purely neural
processes, although we suggest this is less likely.
Finally, there are a number of limitations that n
The Journal of Neuroscience, January 20, 2016 • 36(3):1008 –1018
Systems/Circuits
Chronic Neuropathic Pain: It’s about the Rhythm
Zeynab Alshelh,1 Flavia Di Pietro,1 Andrew M. Youssef,1 Jenna M. Reeves,1 Paul M. Macey,3 E. Russell Vickers,1
Christopher C. Peck,2 Greg M. Murray,2 and XLuke A. Henderson1
1
Department of Anatomy and Histology and 2
Faculty of Dentistry, University of Sydney, Sydney, New South Wales 2006, Australia, and 3
School of Nursing
and Brain Research Institute, University of California, Los Angeles, Los Angeles, California 90095
The neural mechanisms underlying the development and maintenance of chronic neuropathic pain remain unclear. Evidence from
human investigations suggests that neuropathic pain is associated with altered thalamic burst firing and thalamocortical dysrhythmia.
Additionally, experimental animal investigations show that neuropathic pain is associated with altered infra-slow (0.1 Hz) frequency
oscillations within the dorsal horn and somatosensory thalamus. The aim of this investigation was to determine whether, in humans,
neuropathic pain was also associated with altered infra-slow oscillations within the ascending “pain” pathway. Using resting-state
functional magnetic resonance imaging, we found that individuals with orofacial neuropathic pain have increased infra-slow oscillatory
activity throughout the ascending pain pathway, including within the spinal trigeminal nucleus, somatosensory thalamus, thalamic
reticular nucleus, and primary somatosensory cortex. Furthermore, these infra-slow oscillations were temporally coupled across these
multiple sites and occurred at frequencies similar to calcium waves in activated astrocytes. The region encompassing the spinal trigeminal
nucleus also displayed increased regional homogeneity, consistent with a local spread of neural activity by astrocyte activation. In
contrast, no increase in oscillatory behavior within the ascending pain pathway occurred during acute noxious stimuli in healthy
individuals. These data reveal increased oscillatory activity within the ascending pain pathway that likely underpins increased thalamocortical
oscillatory activity, a self-sustaining thalamocortical dysrhythmia, and the constant perception of pain.