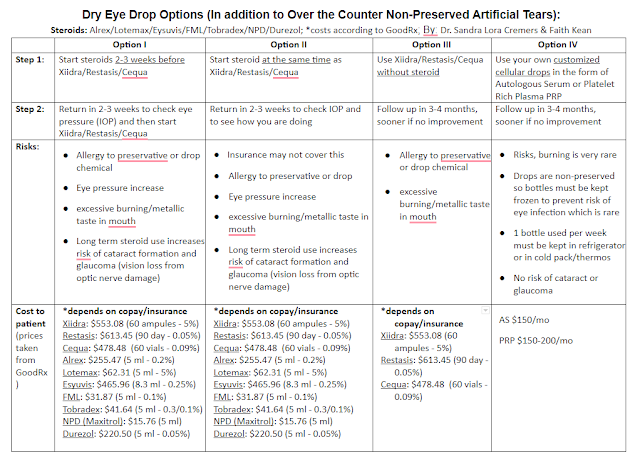
The key FDA-approved dry eye drops are Restasis, Xiidra, and Cequa. They are drops that decrease inflammation. They are all non-preserved. They are all used 2x/day (though some MDs will recommend them “off-label” 3-4 times per day for severe dry eye patients. They have their benefits and side effects. They all come in containers like below which can be hard to use for older patients.
Restasis is 0.05% cyclosporine and the oldest FDA approved drop for aqueous deficiency (just the water/aqueous part of the tear) dry eye. It takes about 3months for blood levels to achieve to feel improvement in symptoms in many patients. This drop can cause significant eye-burning when applied, even after the initial 3 months, and allergy.
Cequa is a new version of cyclosporine 0.09% and takes about 28 days for symptom improvement according to FDA trials. This drop can cause significant eye-burning when applied, even after the initial days and allergy.
Xiidra is an LFA-1 antagonist and usually works in 2-3weeks but can cause significant burning and a metallic taste in the mouth after use (especially if the puncta [the canal between the eye and nose on corners of eyelids] is open). Allergy to Xiidra is not uncommon. More information about these FDA-approved drops below.
Autologous Serum and Platelet Rich Plasma are cells used from your own blood which provide customized cellular therapy to help decrease inflammation and heal cells and tissue. There is rarely any side effects, such as burning. AS and PRP have been used for years on patients with dry eyes and usually costs about $150-250/month.
Restasis: is an emulsion; white opaque to slightly translucent. It contains:
- Cyclosporine 0.05%
- Glycerin
- Castor oil
- Polysorbate 80
- Carbomer copolymer type A
- Purified water
- Sodium hydroxide
1% to 5% of patients had:
conjunctival hyperemia, eye discharge, tearing/epiphora, eye pain, foreign body sensation, itching/pruritus, stinging, blurry vision, visual changes
Post-marketing Experien hypersensitivity (including eye swelling, urticaria, rare cases of severe angioedema, face swelling, tongue swelling, pharyngeal edema, and dyspnea); and superficial injury of the eye (from the vial tip touching the eye during administration).
Cequa is a solution; clear, colorless ophthalmic solution. It contains:
- Cyclosporine 0.09%
- Polyoxyl hydrogenated castor oil
- Octoxynol-40
- Polyvinylpyrrolidone
- Sodium phosphate monobasic dihydrate
- Sodium phosphate dibasic anhydrous
- Water for injection
- Sodium hydroxide
References: