The Meibomian glands are large sebaceous glands that are located as separate gland strands in parallel lines within the tarsal plates of the each eyelids
. Each lower lid has approximately 25 glands and each upper lid has approximately 40 glands. The upper lid glands have more oil than the lower lid glands in total volume.
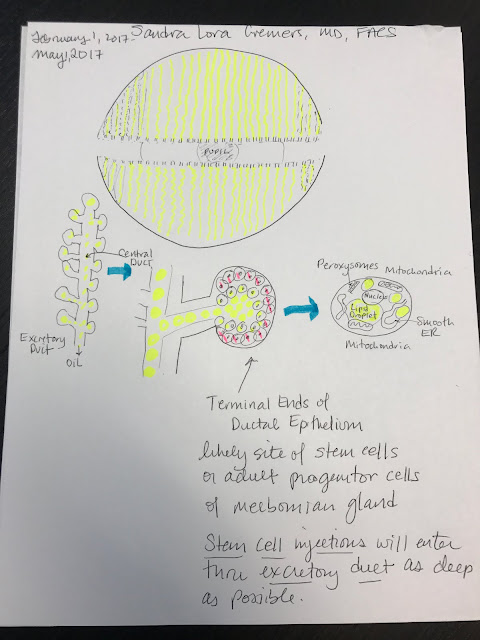
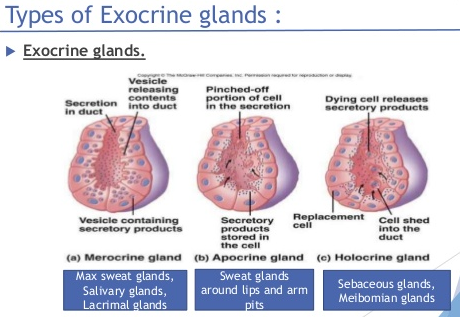 Their oily product (meibum) is secreted by a holocrine mechanism during which the cells (meibocytes) are burst and form meibum after synthesis and accumulation of lipids inside its cell (see my hand drawing above..). Meibum is made up of:
Their oily product (meibum) is secreted by a holocrine mechanism during which the cells (meibocytes) are burst and form meibum after synthesis and accumulation of lipids inside its cell (see my hand drawing above..). Meibum is made up of:
77% wax and sterol esters: including fatty acids, fatty alcohols, and cholesterol
8% phospholipids
9% digylcerides, triglycerides and hydrocarbons
Meibocytes located in acini (a small saclike cavity in the gland, surrounded by secretory cells). Meibocytes are secretory cells that are responsible for the production of meibum. During maturation, the meibocytes’ nuclei shrink and disintegrate, forming the oily product. Meibocyte production should be constant, which accounts for the continual secretion of oil. If you blink, the muscles of your eyelid margin and around the eye contract to squeeze out the oil from the orifice of the meibomian gland. This likely creates a negative pressure which induces the forward moving push for more oil. This intern stimulates the production of more oil from the meibocytes. Likely stem cells at the base of the acini produce more and more meibocytes as long as the conditions are favorable (ie, a low inflammatory state, a good hormonal balance, maybe even the right temperature and humidity) and the oil is pumping out sending a signal that more oil is needed.
After production in the gland acini, meibum is transported through the ductal system via the connecting duct (ductule) and the central duct towards the orifice at the free lid margin close to the inner lid border. The embryological development of the Meibomian glands takes place during the differentiation of the eyelids in the sealing phase of eyelids development. They are not directly associated with hair follicles but share important similarities in embryology, structure and keratinization potency with the cilia. Similar to the sebaceous glands Meibomian glands are regulated via sex hormones and androgens and need a good nerve supply to continue to work properly. Studies show that in some patients, estrogens act antagonistically to lipid production.
However, in contrast to other sebaceous glands they also have a distinct innervation, apart from sympathetic and sensory primarily by parasympathetic fibers that share the innervation pattern of the lacrimal glands.
Meibomian Glands have stem cells in them, but there are few of them and very hard to isolate. A study using the H2B-GFP/K5tTA mouse suggests that the terminal ends of the ductal epithelium are potential sites of adult progenitors or stem cells of the meibomian gland (Parfitt et al., 2015), rather than the central duct or peripheral acinar cells as has previously been suggested (Lavker, 2003, Olami et al., 2001).
THE MATURATION OF MEIBOCYTES TAKES ABOUT ONE AND A HALF WEEKS
The time of a meibocyte, from the start of maturation at the basement membrane until it disintegrates in the center of the acinus, takes roughly around one and a half week, as observed by OLAMI and colleagues in the rat, but may be slightly different in different species.
Since each Meibocyte is lost during the secretory process but the acinus does not end its existence after one ´round´of lipid production and instead continuously produces lipids, we must further assume that there should be a replacement of the meibocytes that are lost in action. This is indeed true, because in the basal cell layer in the periphery of the acinus on the basement membrane, there are stem cells that continuously divide and constitute new cells that start the maturation process.
BEST WAY TO ANALYZE MEIBUM FROM THE MEIBOMIAN GLANDS
The spatula method of collection seems to be the best. The highest mean quantity of cholesterol detection was associated with spatula collection, as well as the lowest mean quantity of inorganic phosphates. Although inorganic phosphate was detected in 40% of samples collected with this technique, the lowest mean quantity of inorganic phosphate was associated with spatula collection of all four techniques in the current study. Spatula collection was second only to the Dacron swab in terms of association with mean quantity of total lipid detected, without the substantial prospective sources of device contamination. Of additional benefit will be results from planned analysis of samples by mass spectrometry to determine with higher specificity the composition of the samples as well as the collection devices.
References:
Sullivan D. Androgen Influence On The Meibomian Gland. Investigative Ophthalmology & Visual Science. 2000;41(12):3732-42.
Optom Vis Sci. 2011 Apr;88(4):525-33. doi: 10.1097/OPX.0b013e318214ac0f.
Examination of human meibum collection and extraction techniques.
Abstract
PURPOSE:
To compare various meibum collection methods and extraction techniques.
METHODS:
Sixty subjects, all successful contact lens wearers, were seen on two visits. Meibum was collected from the lower lid of the right eye with a glass microcapillary tube, and with a Dacron swab, cytology microbrush, or spatula from the left eye. Extraction with 2:1 chloroform:methanol was done either immediately or after data collection was complete. Individual samples were divided into four equal aliquots for analysis of total lipids, cholesterol, and inorganic phosphates by assay-based techniques. Effects of collection method, extraction, and dry eye status were examined using repeated measures analysis of variance and logistic regression.
RESULTS:
Total lipids showed significance for collection device (p < 0.0001) but not for extraction technique (p = 0.13) or dry eye status (p = 0.97). Dacron swab collection was associated with more total lipid on average than each other collection device (p < 0.0001). The cholesterol assay showed significance of collection device (p < 0.0001) and extraction technique (p = 0.0002) but not dry eye status (p = 0.55). Spatula collection was associated with more cholesterol on average than each other collection device (p < 0.0001). For inorganic phosphates, immediate extraction (p < 0.0001), cytology microbrush collection (p < 0.0001), and non-dry eye status (p = 0.03) were associated with the greater likelihood of detection.
CONCLUSIONS:
Dacron swab collection was associated with the highest average amount of total lipid detected, whereas spatula collection and immediate extraction was associated with the highest average amount of cholesterol detected. Cytology microbrush collection with immediate extraction on non-dry eye subjects was associated with the highest probability of detection of inorganic phosphates.
 Their oily product (meibum) is secreted by a holocrine mechanism during which the cells (meibocytes) are burst and form meibum after synthesis and accumulation of lipids inside its cell (see my hand drawing above..). Meibum is made up of:
Their oily product (meibum) is secreted by a holocrine mechanism during which the cells (meibocytes) are burst and form meibum after synthesis and accumulation of lipids inside its cell (see my hand drawing above..). Meibum is made up of:

