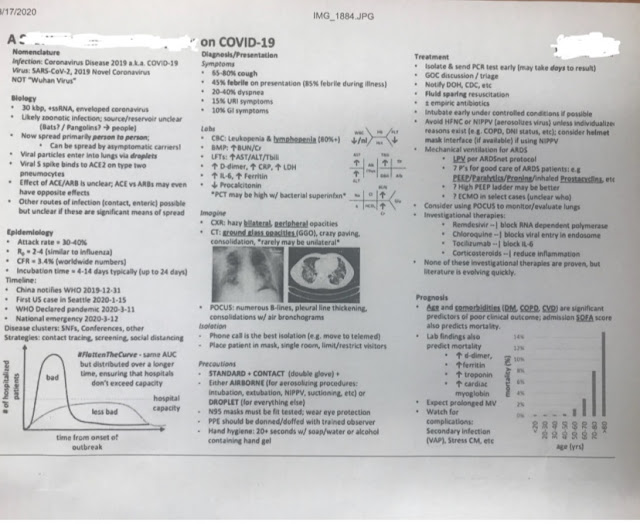
This (below after update) is from a ICU colleague in Seattle. I found this very helpful.
I would add: intermittent fasting and low carb diet helps immune system. As do many suggestions on this previous blog post (like eat more tumeric, curry, Vitamin D, etc): https://drcremers.com/2017/05/best-ways-to-fight-cold-and-from.html
Personally, I have a low threshold –if feel any tingling in nose or sore throat coming on– for gargling & spitting out betadine/povidone iodine 5% (never bleach!) & sticking a cuetip (or rolled up tissue) into nostrils soaked a bit in 5% betadine/povidone or 70% alcohol. Please check with your PCP before doing the below but we have been using 5% betadine/povidone or 70% alcohol for years on the eyeball before surgery as it is very safe for delicate cells.
https://drcremers.com/2017/08/povidone-iodine-is-safe-and-effective.html
Update:
Finally there is a clinical trial on using Povidone Iodine/Betadine to prevent COVID’s viral load and infection. Why is not every university doing this study??
https://clinicaltrials.gov/ct2/show/NCT04364802
COVID-19: Povidone-Iodine Intranasal Prophylaxis in Front-line Healthcare Personnel and Inpatients (PIIPPI)
 |
The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. |
| ClinicalTrials.gov Identifier: NCT04364802 |
|
Recruitment Status : Recruiting
First Posted : April 28, 2020
Last Update Posted : May 14, 2020
|
Sponsor:
Alexandra Kejner
Information provided by (Responsible Party):
Alexandra Kejner, University of Kentucky
- Study Details
- Tabular View
- No Results Posted
- Disclaimer
- How to Read a Study Record
Brief Summary:
Povidone-iodine (PVP-I) is a broad-spectrum antiseptic with activity against bacteria, fungi, and viruses. It has been previously used in both intranasal preparations against Methicillin Resistant Staphylococcus Aureus (MRSA) as well as oral preparations in in-vitro studies of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV), influenza H1N1, and rotavirus with good efficacy. This study will evaluate the efficacy of PVP-I as prophylaxis in Coronavirus Disease 2019 (COVID19)-negative front-line health care workers and hospital patients.
| Condition or disease | Intervention/treatment | Phase |
|---|---|---|
| COVID-19SARS-CoV 2 | Drug: Povidone-Iodine Nasal Spray and Gargle | Phase 2 |
The COVID-19 pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus – 2 (SARS-CoV-2), has been implicated in over 900,000 cases in the United States alone and has been found to affect as many as 28% of healthcare workers (HCW) worldwide. The most current statistics from Iceland, China, and Italy indicate that up to 50% of infected patients may be asymptomatic or with negligible symptomatology. This, added to the national shortage of personal protective equipment (PPE) and the need to reuse PPE, lends to the significantly increased risk to healthcare providers.
The highest concentration of viral particles resides within the nasopharynx. The virus is thought to spread via respiratory droplets with the potential for transmission via inhalation of droplets, contact to the nose and mouth with infected materials, and airborne transmission. Given that frontline workers are involved in high-risk procedures including intubation, bronchoscopy, proning patients (which can lead to droplet production) and in some cases are reusing PPE, finding ways to reduce viral load or viral exposure are paramount.
Povidone-iodine (PVP-I) is a broad-spectrum antiseptic with activity against bacteria, fungi, and viruses. It has been previously used in both intranasal preparations against MRSA as well as oral preparations in in-vitro studies of SARS-CoV, MERS-CoV, H1N1, and rotavirus with good efficacy.
Due to the known breadth of its antiviral activity and similarities in molecular structure, it can be extrapolated that PVP-I should have robust activity against SARS-CoV-2. Eggers et al found that at a concentration of 1% there was a reduction of viral activity of 99.99% in in-vitro assays. At 2 minutes, a concentration of 0.23% was enough to reduce viral loads appreciably.
PVP-I is widely used as an antiseptic and is well-tolerated and has been shown to have little to no effect on mucociliary clearance, olfaction, or thyroid function if iodine holidays are taken.
In this study, front line healthcare workers will be asked to complete a pre-participation survey and screened for COVID positivity. They will then be given premade PVP-I gargles and nasal sprays, as well as a calendar card to mark compliance. PVP-I nasal spray and gargle (10% diluted 1:30) will be used prior to the start of a shift, during “lunch break”, and at the end of shift. First, the nasal spray will be sprayed in the nose (2 sprays each naris). For adequate coverage, the participant should be able to taste the iodine or see it in the back of the throat. This should be left in place for 30 seconds. Then, the participant will gargle the solution for 30 seconds and not have anything to eat or drink by mouth for 30 minutes. Treatment will continue for 3 weeks, or until the healthcare worker presents with COVID symptoms. Participants will then be tested for COVID positivity and asked to fill out a second questionnaire assessing study tolerability. At completion of the study, they will be asked to turn in their calendar card to assess how many applications they were able to complete.
Given the high rate of asymptomatic carriers, a second arm will also be planned for patients who have a 7+ day hospitalization or who are set to undergo a significant surgical procedure. These patients will be offered participation in the study as well and will be given the same questionnaire and undergo preoperative testing if they consent. For patients in the study group, PVIP gargle and nasal sprays will be applied preoperatively or shortly after admission and enrollment in the study for the non-operative group. The patients will then be retested in 2 weeks or as directed by the presentation of symptoms concerning for infection with SARS-CoV-2.
| Study Type : | Interventional (Clinical Trial) |
| Estimated Enrollment : | 250 participants |
| Allocation: | Non-Randomized |
| Intervention Model: | Parallel Assignment |
| Masking: | None (Open Label) |
| Primary Purpose: | Prevention |
| Official Title: | Povidone-Iodine Intranasal for Prophylaxis in Front-line Health-care Personnel and Inpatients During the Sars-CoV-2 Pandemic |
| Actual Study Start Date : | April 29, 2020 |
| Estimated Primary Completion Date : | May 2021 |
| Estimated Study Completion Date : | May 2021 |
Resource links provided by the National Library of Medicine

| Arm | Intervention/treatment |
|---|---|
| No Intervention: Healthcare Workers – Control
Front-line healthcare workers (FLCHW) who are negative for COVID will receive standard PPE and a pre- and post-study test for COVID-19.
|
|
| Experimental: Healthcare Workers – PVP-I
Front-line healthcare workers (FLCHW) who are negative for COVID-19 will receive standard PPE and a pre- and post-study test for COVID-19. Additionally, they will receive PVP-I spray and gargle.
|
Drug: Povidone-Iodine Nasal Spray and Gargle
Healthcare workers will receive standard PPE and a pre- and post-study nasal swab COVID19 test. Additionally, they will receive povidone-iodine nasal spray and gargle (10% diluted 1:30) to use at the beginning of their shift, in the middle, and at the end of their shift.
|
| No Intervention: Inpatients – Control
Inpatients who have a 7+ day hospitalization or who are set to undergo a significant surgical procedure will receive standard care and a pre- and post-study COVID-19 test.
|
|
| Experimental: Inpatients – PVP-I
Inpatients who have a 7+ day hospitalization or who are set to undergo a significant surgical procedure will receive standard care and a pre- and post-study COVID-19 test. Additionally, they will receive PVP-I gargle and nasal sprays that will be applied shortly after admission or perioperatively.
|
Drug: Povidone-Iodine Nasal Spray and Gargle
Patients will receive standard of care treatment and a pre- and post-study nasal spray COVID19 test. Additionally, they will receive povidone-iodine nasal spray and gargle shortly after admission or preoperatively.
|
Primary Outcome Measures :
- Percent of healthcare workers testing positive for COVID-19. [ Time Frame: 3 weeks ]
Percent of healthcare workers that become positive for COVID-19 during the study.
- Percent of patients testing positive for COVID-9. [ Time Frame: 2 weeks ]
Percent of patients that become positive for COVID-19 during the study.
Secondary Outcome Measures :
- PVP-I Ease of Use [ Time Frame: 3 weeks ]
Patients will rate the ease of use for PVP-I treatment on a scale from 1-5 after the initial use. Lower scores indicate increased ease of use (1=”easy”) while higher scores indicate increased difficulty (5=”impossible”).
- PVP-I Comfort [ Time Frame: 3 weeks ]
Patients will rate the comfort of PVP-I treatment on a scale from 1-5 after the initial use. Lower scores indicate increased comfort (1=”not so bad”) while higher scores indicate discomfort (5=”worst pain of my life”).
Other Outcome Measures:
- Adherence to treatment protocol [ Time Frame: 3 weeks ]
Participants will fill out a daily questionaire assessing treatment frequency. Adherence will be calculated as the percent of correct dosing.
Information from the National Library of Medicine

Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
| Ages Eligible for Study: | 18 Years to 99 Years (Adult, Older Adult) |
| Sexes Eligible for Study: | All |
| Accepts Healthy Volunteers: | Yes |
Criteria
Inclusion Criteria:
- healthcare worker OR
- patient with expected hospital stay of 7+ days OR
- patient admitted for major surgery
- COVID19 negative by nasal swab test
- asymptomatic for COVID19
- able to consent
Exclusion Criteria:
- positive for COVID19 by nasal swab
- symptomatic for COVID19
- unable to consent