This is for a dear patient.
Epstein-Barr virus (EBV) and Dry Eye Disease: The Links
It is well known that viruses can trigger inflammatory responses that can lead to chronic dry eye symptoms. Here is the run down of what is known.
1. Dry Eye Disease (DED): is multifactorial, ie do to many causes
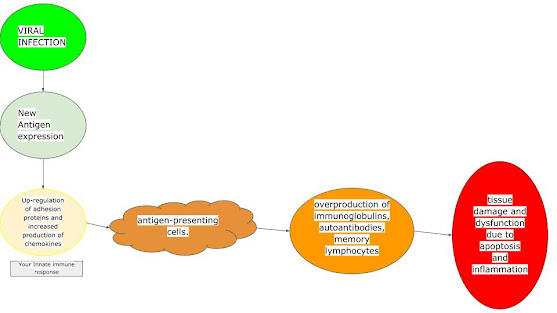
2. Viral infection can induce cause cells to express new antigens on their surface which lead to the production of autoantibodies, cytotoxic T-cell directed to different host tissues: This means virus can cause your own body to start attacking itself.
The exact mechanism is as follows:
3. There are many diseases associated with viral infections
Sjögren syndrome-like illness and associated dry eye symptoms or signs has been associated with:
a. Human T-cell lymphotropic virus (HTLV),
b. Human immunodeficiency virus (HIV),
c. Epstein-Barr virus (EBV),
d. Hepatitis C virus (HCV),
Here is more information from below references:
https://pubmed-ncbi-nlm-nih-gov.proxy1.library.jhu.edu/19028369/
Viral infections related do dry eye
The best-studied viral agents related to systemic infection and DED manifestation are HTLV, HIV, HCV and EBV.
The potential pathogenic mechanisms to DED associated with the viral infection in the lacrimal gland are the below. It is unclear if the virus directly attacks the meibomian gland or if there is a secondary inflammatory scarring process (it could be both).
EBV
EBV (Epstein Barr virus) is herpes virus that infects epithelial cell located in oropharyngeal tissues, salivary glands and B-lymphocytes. The virus occurs worldwide and most people become infected with EBV during the first two decades of life. In the United States, as many as 95% of adults between 35 and 40 years of age have been infected. Primary EBV infection is usually asymptomatic and resolves spontaneously. Occasionally, EBV infection may cause infectious mononucleosis characterized by fever, pharyngitis and general lymphadenopathy(38). After the primary infections it remains latent and occasionally reactivates in salivary glands(39). EBV infection of B cell activates intrinsic pathway that results on continuous cellular division and consequently lymphoproliferation.
There is increasing evidence suggesting that EBV infection may be related to lacrimal gland lymphocytic proliferation of Sjögren syndrome, which leads to decreased aqueous tear production and severe DED(40,41). Many studies have reported primary Sjögren syndrome development immediately after serological confirmation of infectious mononucleosis(42-44). EBV genome has been amplified from the majority of lacrimal gland biopsies of Sjögren patients postulating that EBV may be considered a risk factor for the lacrimal gland pathologic mechanisms of Sjögren syndrome(43). Salivary and lacrimal glands differ in the type of EBC infection observed in biopsies from Sjögren patients. Minor salivary gland shows CD4 T cells infiltration and less severe inflammation. On the other hand, in lacrimal gland biopsies the infiltrating cell type is predominately B-lymphocytes and EBV antigen and EBV DNA are detected in ductal epithelia associated high levels of lymphoproliferation surrounding infected ducts or focci replacing secretory acini(45).
HTLV
The human T-cell lymphotropic virus (HTLV) infection is endemic in Japan, the Caribbean basin, Central and South America and Africa. It is transmitted through sexual intercourse, breast-feeding, blood transfusion and sharing of contaminated syringes and needles. HTLV is characterized by asymptomatic infection in most of seropositive cases. Although 90% of the approximately 20 million infected people worldwide remain asymptomatic carriers during their lives, HTLV infection is also associated with systemic and ocular complications(14).
HTLV infection is etiologically linked to two potential fatal diseases: 1) the malignant proliferation of T cell causing the adult leukemia/lymphoma (ATL) and 2) a neuromyelopathy known as tropical spastic paraparesis (TSP). ATL is considered an aggressive lymphoproliferative malignancy with short survival in its acute form, occurring in less than 5% in HTLV infected people. TSP is a chronic meningomyelitis in the spinal cord, with demyelination and axonal degeneration leading to the development of a slowly progressive spastic paraparesis, high impairment of gait, autonomic dysfunction and profound repercussions on abilities and quality of life of the patients(15,16). HTLV also causes dermatitis, pneumonia, polymyositis, thyroiditis and Sjögren’s like syndrome(16).
HTLV ocular lesions may present as uveitis, dry eye, keratitis and retinal vasculitis(17). The prevalence of uveitis is controversial in different parts of the world. In Japan, for instance, a 35.4% prevalence of uveitis in patients infected with HTLV was observed, while in Martinique, the prevalence was 14.5%(18). The incidence of dry eye in seropositive patients was 36.4% in study conducted in the region of highest prevalence of HTLV infection in Brazil. Moreover, 54.4% of DED in TSP patients and 20.3% in asymptomatic seropositives. In those patients immunophenotyping analysis shown high levels of both CD4+ and CD8+(19). Another study, evaluating 200 infected patient found 37% of DES accompanied by lymphoplasmocytoid infiltration of secondary salivar gland(20,21).
Serological studies demonstrated the prevalence of antibodies to HTLV in Sjögren patients ranging from 23-36%, significantly higher than that among blood donors. Salivary IgA antibodies to HTLV were found in seropositive HTLV patients with SS(22,23). Indeed, two independent studies confirmed the presence of HTLV genome in salivary glands samples from patients with Sjögren(24,25).
HIV
HIV (human immunodeficiency virus) infection may present with a large variety of primary and secondary (caused by opportunistic infections) ocular manifestations, some of those have threatening potential to vision and life quality. The incidence and presentation of the AIDS epidemic and its correlated complications have dramatically changed since the introduction of the potent antiretroviral therapies (also know as highly active antiretroviral therapy, or HAART), but remains as a dramatic public health in very low income countries.
DED appears to be much more prevalent among individuals with AIDS (21.4-38.8% of HIV-infected men, 16.9% of HIV-infected women) than in the general population(13,26,27). Burtin et al. evaluated the ocular surface and DED complains in a group of HIV positive patients. According to this study, 70% of them had complained of DED symptoms, 85% present at least one clinical sign of ocular surface dysfunction tested through Schirmer test, tear break-up time and lissamine stain and the impression cytology revealed a decrease in the number of dendritic cells(28).
Geier et al. showed that decreased tear production occurs in approximately 20% to 25% of patients with HIV infection without correlation with the CD4+v lymphocites blood count, or to the severity of HIV disease, in a group of 144 HIV patients(29). Although the entire pathogenesis of the aqueous tear deficiency in HIV-infected remain unclear, it may be associated with lymphocytic infiltration and destruction of the lacrimal gland acini and ducts.
In fact, a Sjögren’s syndrome-like picture may be present in HIV -infected patients who develop the diffuse infiltrative lymphocytosis syndrome (DILS). Thus, it is an exclusion criteria for individuals under investigation for Sjögren syndrome(30). DILS is a disorder in patients with HIV infection that is characterized by the enlargement of salivary and lacrimal glands and a varying intensity of DED symptoms. In addition, DILS is accompanied by persistent circulating and infiltration of CD8-positive lymphocytes. DILS may mimic Sjögren’s syndrome in terms of symptoms and parotid and lacrimal glands involvement. On the other hand, it differs by the high frequency of extra glandular sites of lymphocytic infiltration, such as lungs, muscles and liver, scarcity or absence of serum autoantibodies and the nature of infiltrating lymphocytes. While in Sjögren syndrome presents lymphocytic infiltration of CD4+ and in DILS it is a CD8+ lymphocytes and anti-Ro and anti-La are seen less frequently(2,31). The prevalence of DILS had dropped significantly after the introduction of HAART, indirectly inferring that the HIV infection contributes to DILS pathogenesis.
Hepatitis C
Hepatitis C virus (HCV) is frequently associated with autoimmune features and extra hepatic manifestations, especially with chronic infection(32). The prevalence of HCV infection in patients with primary SS have been analyzed in different studies and its is comparatively higher than in the general population although varying geographically(3,33,34).
Ocular manifestations such as retinopathy, scleritis and keratitis have been well documented(35). Cacoub et al. evaluating a group of 312 HCV patients found xerostomia and/or xeroftalmia symptoms in 10%(36) which makes the ocular surface one of the most important sites of manifestations in HCV infected patients(37).
Both entities, Sjögren syndrome and HCV, are characterized by B-cell hyperactivity although related to different etiologies, autoimmune and infectious respectively. HCV have demonstrated capability to infect and replicate in the salivary and lacrimal gland tissues leading to lymphocytic infiltration, signs and symptoms of SS. Patients with Sjögren syndrome associated to HCV infection have demographic, immunological and clinical different profiles. Comparatively, Sjögren syndrome-HCV patients are older aged, higher incidence of extra glandular manifestations, especially cryoglobulinemia, and have negative Ro/La antibodies and hypocomplementemia.
Again the American European criteria for Sjögren syndrome, exclude Sjögren diagnosis in patients who are HCV positive(30).
HSV and ocular surface
HSV-1 (herpes simplex virus-1) ocular infection is a common cause of ocular surface disorder. It can present in a broad range of manifestations from primary blepharoconjuntivitis to recurrent forms of keratitis and even intraocular involvement as that seen in retinitis and uveitis(46,47). Although there is no evidence of HVS direct infection in the lacrimal gland it has been demonstrated that corneal sensation and tear production are significant lower in patients with ocular herpetic disease(48,49).
CONCLUSIONS
Circumstantial evidence suggests that systemic and ocular viral infections, along with many environmental and other risks factors, may play an important role in the pathogenesis of dry eye disease. Patients with moderate to severe dry eye disease and other clinical manifestations should be investigated by serology of mentioned viral systemic infections. Many studies confirmed the association of viruses and lacrimal gland dysfunction and many others have been addressed efforts to the understanding its mechanisms. Future research should characterize this subpopulation, the application of diagnostic tools and the possible benefits of specific antiviral treatment as a therapeutic approach for dry eye disease.
Finally, the present review reinforces the importance of ophthalmologists as one of the healthcare professional able to diagnose a potentially large number of infected patients with high prevalent viral agents. Moreover, it may contribute to make more widely known the possible association between viral infections and dry eye disease and the importance of including an ophthalmologic evaluation as part of the medical approach to patients infected with specific chronic viral infections.
The role of Epstein-Barr virus infection in primary Sjögren’s syndrome
- PMID: 31356378
- DOI: 10.1097/BOR.0000000000000622
Abstract
Purpose of review: The purpose of this article is to draw attention to the role of Epstein-Barr virus (EBV) virus in the pathogenesis of the primary Sjögren’s syndrome. The article introduces the problem of consequences of EBV acute infection, and its reactivation, in association with the immune response modulation by the virus and with an increased risk of developing systemic autoimmune diseases and EBV-associated cancers.
Recent findings: The knowledge about the mechanisms by which the virus may stay for years in a latent phase, unrecognized by the host response immune cells is constantly expanding. There are several mechanisms and theories about EBV influence on the autoimmune process in Sjogren’s syndrome (pSS), including the similarity (molecular mimicry) between viral EBNA-2 protein and Ro-60 antigen or EBER-1 and EBER-2 viral proteins and La antigen.
Summary: The influence of EBV infection on the development and course of pSS has been proven. It has also been established that both EBV and pSS result in the increased risk of tumor (especially lymphoma) development. In the light of these findings, new ways to manage EBV infections are being sought. Optimal methods for assessing EBV infection status are being devised. Research also aims at finding therapies, which target EBV through the inhibition of the autoimmune process and of viral activity. The present article is an attempt to discuss the most important phenomena and elements linking EBV infection to the primary Sjögren’s syndrome.