Popular eye drops, such as Visine, promise to “get the red out,” but often clamp down the blood vessel circulation which affects cells and the tear film, and eventually can make your eyes feel even drier and make them look more red: often called “rebound redness.”
The ingredient that causes this is Tetrahydrozoline which is a sympathomimetic amines. It works by temporarily narrowing the blood vessels in the eye. This narrowing occurs in all blood vessels as it does not discriminate between “ugly” blood vessels and normal vessels. The rebound redness occurs as a response of the body to this narrowing as well as the direct toxic effect of this chemical to the surface cells of the eye over chronic use of the drug. Avoid Tetrahydrozoline as much as possible.
However, even Visine artificial tears, which have the preservative BAK: benzalkonium chloride, can cause microdamage to eye cells which can lead to problems in the future for some patients
Active Ingredients:Glycerin 0.2% (Lubricant)Hypromellose 0.2% (Lubricant)Polyethylene glycol 400 1% (Lubricant). Inactive ingredients:ascorbic acid, benzalkonium chloride, boric acid, dextrose, glycine, magnesium chloride, potassium chloride, purified water, sodium borate, sodium chloride, sodium citrate, sodium lactate.
This is why non-preserved or preservative-free tears are best.
Retaine remains the most loved non-preserved/preservative free eye drop among my patients.
Rarely does a patient not like Retaine but I have seen some patients who say it makes them feel worse or does not help.
But there are many artificial tear options.
I try, as much as possible, to slip in Omega 3 into our diet. I will slip in flax seed & chia seeds into cake & cookie recipes if I have to make them for a school event. I slip it into quiches and other foods that my kids do not want to know about. We try to eat a lot of wild salmon when it is in season. We eat canned herring from Amazon which is edible with an instant pot recipe I make every month: otherwise the kids do complain. I try to feed them walnuts which have Omega 3.
I prefer liquid versions of Omega 3.
I have also used these for the kids:
Other studies have shown that combinations of Primrose Oil, Vitamin B6, Vitamin C work to help with dry eyes. They are small studies with no control groups, but the risks of these combinations are so low, it is worth a try before trying steroids and maybe even other prescription dry-eye drops in my opinion.
In another study: patients suffering from dry eye symptoms who exhibited a chronic need of artificial tears were given
1. 2 capsules of 500 mg each of evening Primrose oil (Efamol-73% linoleic acid and 10% gamma-linolenic acid),
2. 50 mg vitamin B6 (pyroxidine) and
3. 1 g vitamin C
Three times a day.
Over 50% of those in the study showed substantial improvement within 2-6 weeks.
Again this is a small study & am looking for a better study to prove it’s effects. I could not find 1 pill with all 3 items in one.
SLC:
The full study is below: **
Dry eye or Keroconjunctivitis Sicca and other surface disorders is usually a chronic condition. We have no cure. The remedies should focus on 2 aspects:
1. Saving all eye surface cells and meibomian gland cells
2. Palliative care: meaning treat the symptoms to avoid chronic inflammation which can show itself as chronic redness, irritation, burning.
, with the administration of lubricating drops. Current topical products are formulated to both lubricate the eye and enhance certain characteristics of the tear film. For example, hypotonic solutions reduce osmolarity and mucolytic agents can decrease the symptoms of excess mucin strands. Other additives may help lower tension at the water-oil interfaces and mimic some actions of the mucin network. However these palliative measures are temporary and do not address the underlying causes. For example, reduced osmolarity upon instillation may last only about 10 minutes.
- Linoleic Acid
- Gamma Linolenic Acid
- Di-hommo Gamma Linolenic Acid
- PGE1
- Di-hommo Gamma Linolenic Acid
- Gamma Linolenic Acid
**
TREATMENT OF THE SICCA
SYNDROME AND SJOGREN’S SYNDROME
WITH E.F.A., PYRIDOXINE AND VITAMIN C
D. F. HORROBIN, A. CAMPBELL, C. G. MCEWEN
P.O. Box 10 Nun’s Island, Montreal H3E lJS, Canada I Hairmyres Hospital, East Kilbride 2
and Glasgow Eye Infirmary, 3 San@ford Place 3, Glasgow, Scotland
INTRODUCTION
In Sjogren’s syndrome and the sicca syndrome, there is failure of normal secretion of
tears and saliva. ~ In Sjogren’s syndrome, this failure is associated with a “connective
tissue” disease, often rheumatoid arthritis, while in the sicca syndrome the eye abnormalities
may occur either alone or coupled with loss of saliva. Although many therapies
have been tried, none is effective and the patients must resort to frequent use of lubricant
eye drops and lubricant mouth washes, Patients with either syndrome cannot wear
contact lenses and have difficulties watching television, eating and speaking.
A number of observations suggest that the failure of tear secretion may be related to
inadequate formation of prostaglandin (PG) E13: (1) Deficiency of essential fatty acids
causes lacrimal gland atrophy in animals. 1 (2) Deficiency of pyridoxine which seems
important in conversion of linoleic acid to dihomogammalinolenic acid (DGLA) causes
lacrimal gland atrophy. 7 (3) In humans, lack of vitamin C which seems important in
PGEx formation s caused features of Sjogren’s syndrome to appear in 5/5 individuals who
went on a vitamin C deficient diet. 2 (4) The animal model of Sjogren’s syndrome is the
NZB/W mouse. Many of the pathological features in these animals can be prevented by
PGE~ treatment. ‘~ (5) Of 8 PGs tested, only PGE1 had a significant effect in increasing
tear production in rabbits. 6 It, therefore, seemed worth attempting to treat the dry eye
syndrome with essential fatty acids, pyridoxine and vitamin C. One of us (AC) had
previously tried to treat the syndrome with high doses of vitamin C alone with no
SuCCeSS.
CLINICAL REPORTS
Patients were selected for the study on the basis of failure of tear secretion, objectively
demonstrated and necessitating frequent use of lubricant eye drops. Patients were
assessed by clinical observation, by objective measurement of the rate of tear production
using the Schirmer test, by subjective reporting and by changes in the use of lubricant eye
drops. Seventeen patients have been entered into the study to date. Each patient has
received 2 x 500 mg capsules of evening primrose oil (Efamol) containing 73% of linoleic
acid and 10% of ~-linolenic acid, 50mg of pyridoxine and 1 g of vitamin C 3 times per
day. In nine of the patients, the dry eyes were associated with some form of connective
tissue disease (Sjogren’s syndrome) while the other 8 had various forms of the sicca
syndrome. In 3 of the 8, the sicca syndrome had been induced by use of the E-blocking
drug, practolol.
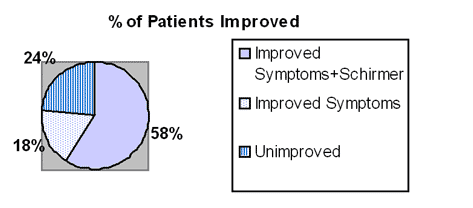
To date, 10 patients have shown both subjective and objective evidence of either
substantial improvement or complete resolution of the dry eye syndrome. One patient
who had been unable to undergo necessary ophthalmic surgery because of absence of
tears has been successfully operated upon. All 3 practolol patients were in this group of
10. The improvement took 2-6 weeks to become apparent and appeared to continue
progressively over several weeks. In 3 patients, although there was no increase of tear
production on Schirmer testing, the eyes appeared moist, the patients felt better and they
253
254 D.F. Horrobin, A. Campbell and C. G. McEwen
reported decreased use of eye drops. In 2 patients, there was objective evidence of
increased tear production but this was not accompanied by any subjective sense of
improvement. Two patients have shown no change after 2 months therapy. Two patients
who had suffered for many years from poor nails which broke frequently, reported a
sharp improvement in nail quality during the treatment.
CONCLUSIONS
In the majority of patients with the dry eye syndrome, the combination of evening
primrose oil, pyridoxine and vitamin C produced a substantial improvement. This is the
first report of a safe, systemic therapy for this disease. Some of the patients are still
improving. The syndrome is often associated with fibrosis and degeneration of the lacrimal
glands. It is possible that in some individuals this has progressed to an irreversible
stage while in others many months of treatment may be required before recovery.
To date, only one dose regime has been tested. It is possible that other doses of one or
other of the components of the regime may be more successful than reported here.
Further work is in progress to determine the optimum dose levels to be used. Once this
regime has been worked out, a placebo-controlled double blind trial will be set in
motion.
REFERENCES
I. AL^M, B. S. and ALAM, S. Q. Proc. Soc. Exp. Biol. Med. 161, 199-203 (1979).
2. Hoot), J., BURNS, C. A. and Hot)o~, R. E. New Enol. 3. Med. 282, 1120-1124 (1970).
3. HoaaoaiN, D. F. and C^MPaELL, A. Med. Hypothese~ 6, 225-232 (1980).
4. KRAK^UER, K., TOR~tEV, S. B. and ZURIER, R. B. Clin. Immunol. Immunopathol. II, 256-264 (1978).
5. MA~<u, M. S., OKA, M. an~ H~p,l~o9i~, D. F. Prostaglandins Med. 3, I19-126 (1979).
6. PHOI.PP, A~OOL, C. Prostaolandins Med. 3, 185-192 (1979).
7. SHI~.aN, M. A. Sjooren’s Syndrome, W. B. Saunders, Philadelphia, 1971.
Ref:
https://www.sciencebasedhealth.com/Webpage.aspx?WebpageId=74
4.2. Omega-6 formulations
A 6-month uncontrolled open-label trial of a combination omega-3 and -6 (1000 mg linseed and 500 mg evening primrose oil) suggested improvement in symptoms and tear stability; the results are unfortunately subject to significant bias as neither the investigator nor the patients were masked and no placebo control was used (Jackson et al., 2011). A 3 month RCT involving a sea buckthorn oil from the sea buckthorn berry (245 mg LA, 149 mg ALA, 346 mg palmitoleic acid (omega-7), 316 mg oleic acid, 108 mg cis-vaccenic acid (omega-7), 6.8 mg vitamin E, 1.8 β-carotene & zeaxanthin) was recently conducted in a cohort of 100 Finnish patients. Although there were no differences between the two groups, there was less increase in tear osmolarity and reduced symptoms in the test than the control group (Larmo et al., 2010). This indicates that sea buckthorn oil treatment minimized the worsening of the clinical signs and symptoms rather than improved them.














