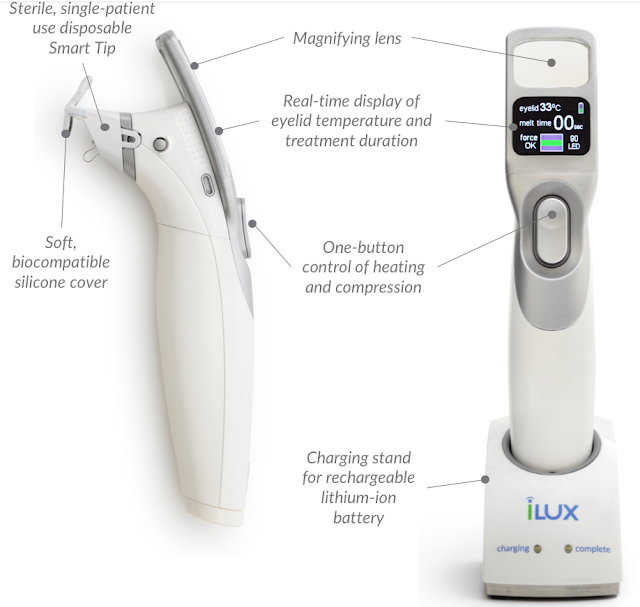
This new device debuted at ASCRS in April 2018 with a price tag of about $8000-8500 per device. The idea is that eye MDs and eye ODs will buy this device and sell the procedure to patients.
Of course, this is not covered by insurance.
It is not FDA. Why? I do not know why the Lipiflow needed FDA approval & this device does not.
Does it save glands? It is not clear as there have not been any head to head studies to prove that it does. I hope it does, but that is not proof yet.
Will we offer this at Visionary Eye Doctors?
I am not sure.
Dry Eye Patients are sick and tired of paying for yet another non-covered procedure. But what are our options? Probing & expression is painful and expensive: even if it works. A non-painful procedure that can prevent one from loosing more meibomian glands is priceless. If anyone has tried it & it really relieved pain, please let me know!
SLC