Tacrolimus (Protopic) has been used to treat dry eye disease in Sjögren syndrome. Tacrolimus, a macrolide produced by Streptomyces tsukubaensis which was discovered in 1984 in Japan while searching for new immunosuppressive and cancer chemotherapeutic agents. It is effective in treatmenting of immune-mediated diseases, including Sjögren syndrome, Atopic Dermatitis, other dermatitis (as pills or ointment) and more:
Tacrolimus is often prescribed for T cell-mediated diseases such as:
1. eczema or atopic dermatitis
2. psoriasis
3. Vitiligo
Orally Tacrolimus is used to:
1. prevent organ rejection
2. Uveitis after Bone Marrow transplantation
3. Minimal change disease of the kidney
4. Kimura’s disease
There is an ongoing investigation to see which one is better: Tacrolimus 0.03% (FK506) eye drops versus Cyclosporine 0.05% eye drops in treatment of dry eye in Secondary Sjogren Syndrome.
Results are pending.
There are risks to use, including a risk of cancer if taken orally, so read below as well if considering its use.
In the meantime, here is an article from 2012 on the use of 0.03% Tacrolimus drops for dry eye.
SLC
Cornea. 2012 Aug;31(8):945-9. doi: 10.1097/ICO.0b013e31823f8c9b.
Clinical treatment of dry eye using 0.03% tacrolimus eye drops.
- 1
- Department of Ophthalmology, School of Medicine, University of São Paulo (Hospital das Clínicas of da Universidade de São Paulo), São Paulo, Brazil.
Abstract
PURPOSE:
To report the clinical outcome of the treatment of dry eyes using 0.03% tacrolimus eye drops (olive oil + tacrolimus 0.03%) (Ophthalmos, Sao Paulo, Brazil).
METHODS:
Sixteen eyes of 8 patients with Sjögren syndrome dry eyes (age, 51.13 ± 9.45 years) were enrolled in this study (prospective noncontrolled interventional case series). Patients were instructed to use topical 0.03% tacrolimus eye drops twice a day (every 12 hours) in the lower conjunctival sac. Schirmer I test, break-up time, corneal fluorescein, and rose bengal staining score were performed in all patients 1 day before, and 14, 28, and 90 days after treatment with 0.03% tacrolimus eye drops.
RESULTS:
The average fluorescein staining and rose bengal staining scores improved statistically significantly after 14 days of treatment and improved even more after 28 and 90 days. The average Schirmer I test did not improve statistically significantly after 28 days of treatment, although we did observe a significant improvement after 90 days of treatment with 0.03% tacrolimus eye drops. The average break-up time did not improve statistically after 14 days of treatment, although we observed a significant improvement after 28 and 90 days of treatment with 0.03% tacrolimus eye drops.
CONCLUSIONS:
Topical 0.03% tacrolimus eye drops successfully improved tear stability and ocular surface status in patients with dry eyes.
I wanted to add all the risks of Tacrolimus (Protopic) in the literature for patients who have been prescribed this by their dermatologist.
Wilkipedia has a good review below as well: https://en.wikipedia.org/wiki/Tacrolimus
Tacrolimus (i.e., fujimycin or FK-506): Chemically tacrolimus is a 23-membered macrolide lacton macrolide (similary to many known antibiotics) that was first discovered in 1987 from the fermentation broth of a Japanese soil sample that contained the bacterium Streptomyces tsukubaensis. It is an immunosuppressant used for liver transplant rejection prophylaxis and is under investigation for use in kidney, cardiac, pancreas, small bowel, and bone marrow transplantation. It is also used in a variety of autoimmune disease. Its mechanism of action is similar to that of cyclosporine, but with 10- to 100-fold greater potency.
In ophthalmology, topical tacrolimus is used in immune-mediated conditions to decrease inflammation, such as:
-ocular allergy: especially atopic conjunctivitis and blepharitis
-graft-versus-host disease,
-corneal transplantation,
-ocular pemphigoid.
-ocular Sjögren’s syndrome (used as 0.03% ophthalmic solution)
-keratoconjunctivitis sicca (used as 0.03% ophthalmic solution)
The risks include below (from Wiki):
I could not find any paper saying Tacrolimus worsens meibomian gland dysfunction.
Side effects[edit]
By mouth or intravenous use[edit]
Side effects can be severe and include infection, cardiac damage, hypertension, blurred vision, liver and kidney problems (tacrolimus nephrotoxicity),[13] hyperkalemia, hypomagnesemia, hyperglycemia, diabetes mellitus, itching, lung damage (sirolimus also causes lung damage),[14] and various neuropsychiatric problems such as loss of appetite, insomnia, posterior reversible encephalopathy syndrome, confusion, weakness, depression, vivid nightmares, cramps, neuropathy, seizures, tremors, and catatonia.[15]
In addition, it may potentially increase the severity of existing fungal or infectious conditions such as herpes zoster or polyoma viral infections.[11]
Carcinogenesis and mutagenesis[edit]
In people receiving immunosuppressants to reduce transplant graft rejection, an increased risk of malignancy (cancer) is a recognised complication.[11] The most common cancers are non-Hodgkin’s lymphoma[citation needed] and skin cancers. The risk appears to be related to the intensity and duration of treatment.
Topical use[edit]
The most common adverse events associated with the use of topical tacrolimus ointments, especially if used over a wide area, include a burning or itching sensation on the initial applications, with increased sensitivity to sunlight and heat on the affected areas. Less common are flu-like symptoms, headache, cough, and burning eyes.[16]
Cancer risks[edit]
Tacrolimus and a related drug for eczema (pimecrolimus) were suspected of carrying a cancer risk, though the matter is still a subject of controversy. The FDA issued a health warning in March 2005 for the drug, based on animal models and a small number of patients. Until further human studies yield more conclusive results, the FDA recommends that users be advised of the potential risks. However, current practice by UK dermatologists is not to consider this a significant real concern and they are increasingly recommending the use of these new drugs.[17]
Canadian Medical Association
Eczema drugs tacrolimus (Protopic) and pimecrolimus (Elidel): cancer concerns
Eric Wooltorton
Reason for posting: Many patients with eczema, or atopic dermatitis, are prescribed the topical immunomodulators tacrolimus and pimecrolimus. The drugs are often given to people for whom the potential side effects of topical corticosteroids (e.g., systemic absorption, skin thinning, telangiectasia) are a concern. However, the US Food and Drug Administration (FDA) recently reviewed the safety of these agents and warned that they may be associated with a risk of cancer.1
The drugs: Tacrolimus and pimecrolimus bind and inactivate calcineurin (a calcium- and calmodulin-dependent serine and threonine phosphatase) and may act by inhibiting T-lymphocyte activation, down-regulating numerous interleukins, interferon-γ, granulocyte-macrophage colony-stimulating factor and tumour necrosis factor-α, and affecting the function of mast cells, basophils and Langerhans cells.
Both agents are more effective than placebo in treating atopic dermatitis. Tacrolimus (0.03% and 0.1% preparations) is more effective than mild topical steroids, and the 0.1% preparation is as effective as more potent topical steroids.2 In contrast, pimecrolimus is less effective than potent steroids (0.1% betamethasone valerate), but its efficacy relative to mild corticosteroids is unclear.2
Common adverse effects include mild, local, temporary burning or pruritus, and users may have increased risk of local varicella-zoster virus infection, herpes simplex infection and eczema herpeticum. Children under the age of 2 receiving topical pimecrolimus had higher rates of respiratory tract infections than children receiving the placebo.1
Lymphadenopathy, usually transient and related to underlying infections, has been reported. However, patients taking systemic tacrolimus (as an immunosuppressive agent after liver and organ transplantation) have reported lymphomas and solid organ tumours, possibly because their defences against cancer have been suppressed.3Only small amounts of the drugs are usually absorbed through the skin; however, some children given topical tacrolimus have blood levels of the drug similar to those given its systemic form.1
Animals (mice, rats, monkeys) given high doses of the drugs topically or orally have a risk of cancer that is dependent on both the duration and dose of the drug.1,3Long-term safety trials involving humans have not been done.
Causative associations are uncertain, but the FDA is also reporting the cases of several patients in whom cancer developed after drug use. For tacrolimus, 19 cases of cancer were reported, involving 16 adults and 3 children under the age of 16. The cancers were diagnosed 21–790 days after the start of therapy (the median time to diagnosis was 150 days). Nine cases involved lymphomas, and 10 involved skin tumours (7 at the site of the drug application). Tumour types included squamous cell carcinoma, cutaneous sarcoma and malignant melanoma. For pimecrolimus, 10 postmarketing cases of cancer were reported, involving 4 children (3 less than 6 years of age) and 6 adults. Of the 10 cases, 6 involved cutaneous tumours and 4 were lymphomas. Diagnoses were made 7–300 days after treatment was started (median time to diagnosis was 90 days).
What to do: As second-line agents, these drugs should be used only if other therapies (topical corticosteroids, emollients) are ineffective or inappropriate. They should not be used by patients with weakened or compromised immune systems, by children under the age of 2 or by patients with active viral skin infections. Short-term or intermittent use is advised. Unfortunately, atopic dermatitis is an uncomfortable, common and chronic condition. Patients should be warned of the potential cancer risk and carefully monitored clinically when taking the drugs. Any patient with nonresolving lymphadenopathy should be appropriately investigated. The lowest concentration of the drugs needed to control a patient’s symptoms should be used. Unnecessary and potentially harmful ultraviolet exposure (from the sun and tanning beds) should be avoided.
Eric Wooltorton Associate Editor, CMAJ
Curr Eye Res. 2017 Nov;42(11):1440-1444. doi: 10.1080/02713683.2017.1339805. Epub 2017 Sep 18.
Tacrolimus Ointment for Refractory Posterior Blepharitis.
Sakassegawa-Naves FE1, Ricci HMM2,3, Moscovici BK2,4, Miyamoto DA3, Chiacchio BB3, Holzchuh R2,3, Santo RM3, Hida RY2,5,3.
Abstract
PURPOSE:
This prospective, randomized, double-blind interventional case series was designed to evaluate the short-term efficacy of 0.03% tacrolimus ointment as a new therapeutic approach for refractory cases of posterior blepharitis.
METHODS:
Forty eyes (20 patients) with posterior blepharitis refractory to previous treatment were randomized. Eighteen eyes (9 patients) were treated with 0.03% tacrolimus ointment and 20 eyes (10 patients) with placebo ointment twice daily. Patients were evaluated with a questionnaire and slit-lamp examination 14 days and 28 days after treatment, and symptoms and signs of blepharitis were compared to those observed at baseline.
RESULTS:
We could observe statistical difference in the outcome measurements of meibomian gland secretion, conjunctival hyperemia, telangiectasia of inferior lid, Rose Bengal, and fluorescein scoring for the study group. As for the symptoms score, we observed statistical difference in the symptoms scoring for pruritus and dry eye sensation in the tacrolimus group.
CONCLUSION:
This study suggests that topical administration of 0.03% tacrolimus ointment can improve some symptoms and some ocular surface status in patients with refractory posterior blepharitis.
Previous post:
Tacrolimus is often prescribed for T cell-mediated diseases such as:
1. eczema or atopic dermatitis
2. psoriasis
3. Vitiligo
Orally Tacrolimus is used to:
1. prevent organ rejection
2. Uveitis after Bone Marrow transplantation
3. Minimal change disease of the kidney
4. Kimura’s disease
Tacrolimus inhibits the production of interleukin-2, a molecule that promotes the development and proliferation of T cells, which are vital to the body’s adaptive immune response (ie, its learned immune response).
It is a 23-membered macrolide lactone that was first discovered in 1987 from the fermentation broth of a Japanese soil sample that contained the bacterium Streptomyces tsukubaensis.
Tacrolimus can cause an allergic contact dermatitis. I have seen about 10 cases but just saw one possibly so I wanted to write about this potential side effect.
Most patients tolerate it very well, though.
SLC
References:
Allergic contact dermatitis from tacrolimus.
- 1
- Department of Medicine, University of California, San Diego, California 92103-8420, USA. dwshaw@ucsd.edu
Abstract
A 9-year-old boy developed allergic contact dermatitis from tacrolimus ointment. Tacrolimus was proven to be the allergen by right-versus-left double-blinded provocative use testing of tacrolimus ointment 0.1% versus inactive vehicle applied twice daily to normal preauricular and antecubital skin. Facial dermatitis appeared after 1 week and antecubital dermatitis after 7 weeks. Furthermore, patch testing of each individual ingredient was positive only with tacrolimus; a concentration of 2.5% in ethanol was required. Forty control patients had negative patch tests with tacrolimus 5% in ethanol. We hypothesize that the unusually long time required to elicit a positive use test on the arm and the high patch test concentration required on the back are caused by low percutaneous absorption through normal extrafacial skin. This is likely to be caused in part by the high molecular weight of tacrolimus. A similar phenomenon may occur when patch testing with neomycin sulfate.
References:
Abstract
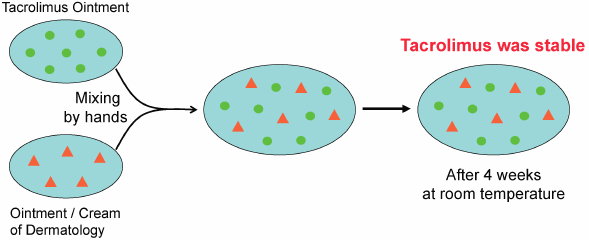
Tacrolimus ointment is used worldwide to treat atopic dermatitis. Although tacrolimus ointment is not suitable for clinical admixtures, it is often mixed with various ointments or creams, such as corticosteroids, antibacterial agents, and moisturizing agents. There is only one report of quality testing of admixtures of tacrolimus ointment with adaparene gel (Differin® Gel). In this study, we used HPLC to evaluate the pharmaceutical stability of tacrolimus mixed with eight different dermatologic ointments or creams. No decrease in the tacrolimus content was observed in any of the mixtures after 4 weeks of storage at room temperature, indicating that tacrolimus admixtures are stable.
References (7)
- 1) Eto T. Usage of topical steroids-propriety of mixed prescription. Jpn. J. Clin. Dermat., 55, 96–101 (2001).
- 2) Japanese Dermatological Association. Guideline of care for the management of atopic dermatitis 2016. Jpn. J. Dermatol., 126, 121–155 (2016).
- 3) Interview Form of protopic ointment 0.1%, Maruho Co., Ltd., 2017.
- 4) Ohtani M. Effect of the admixture of commercially available corticosteroid ointments and/or creams on their efficacy and side effects. Jpn. J. Pharm. Health Care Sci., 29, 1–10 (2003), and references cited therein.
- 5) Ohtani M, Yamaoka Y, Matsumoto M, Namiki M, Yamamura Y, Etoh T. Compatibility of adaparene gel (Differin® Gel) with other kinds of ointments or creams. Yakuzaigaku, 69, 470–476 (2009).
- 6) https://en.wikipedia.org/wiki/Tacrolimus#targetText=Tacrolimus%2C%20also%20known%20as%20fujimycin,the%20risk%20of%20organ%20rejection.
Tacrolimus
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
Medical uses[edit]
Organ transplantation[edit]
It has similar immunosuppressive properties to ciclosporin, but is much more potent. Immunosuppression with tacrolimus was associated with a significantly lower rate of acute rejection compared with ciclosporin-based immunosuppression (30.7% vs 46.4%) in one study.[3] Clinical outcome is better with tacrolimus than with ciclosporin during the first year of liver transplantation.[4][5] Long-term outcome has not been improved to the same extent. Tacrolimus is normally prescribed as part of a post-transplant cocktail including steroids, mycophenolate, and IL-2 receptor inhibitors such as basiliximab. Dosages are titrated to target blood levels.
Ulcerative colitis[edit]
In recent years, tacrolimus has been used to suppress the inflammation associated with ulcerative colitis (UC), a form of inflammatory bowel disease. Although almost exclusively used in trial cases only, tacrolimus has shown to be significantly effective in the suppression of flares of UC.[6][7]
As an ointment, tacrolimus is used in the treatment of eczema, in particular atopic dermatitis. It suppresses inflammation in a similar way to steroids, and is equally as effective as a mid-potency steroid. An important advantage of tacrolimus is that, unlike steroids, it does not cause skin thinning (atrophy), or other steroid related side effects.[8]
It is applied on the active lesions until they heal off, but may also be used continuously in low doses (twice a week), and applied to the thinner skin over the face and eyelids.[citation needed] Clinical trials of up to one year have been conducted. Recently it has also been used to treat segmental vitiligo in children, especially in areas on the face.[9]
Lupus Nephritis
Tacrolimus has been shown to reduce the risk of serious infection in lupus nephritis, when compared to other agents.[10]
Contraindications and precautions[edit]
Contraindications and precautions include:[11]
Topical use[edit]
- Occlusive dressing
- Known or suspected malignant lesions
- Netherton’s syndrome or similar skin diseases
- Certain skin infections[8]
Side effects[edit]
By mouth or intravenous use[edit]
Side effects can be severe and include infection, cardiac damage, hypertension, blurred vision, liver and kidney problems (tacrolimus nephrotoxicity),[13] hyperkalemia, hypomagnesemia, hyperglycemia, diabetes mellitus, itching, lung damage (sirolimus also causes lung damage),[14] and various neuropsychiatric problems such as loss of appetite, insomnia, posterior reversible encephalopathy syndrome, confusion, weakness, depression, vivid nightmares, cramps, neuropathy, seizures, tremors, and catatonia.[15]
In addition, it may potentially increase the severity of existing fungal or infectious conditions such as herpes zoster or polyoma viral infections.[11]
Carcinogenesis and mutagenesis[edit]
In people receiving immunosuppressants to reduce transplant graft rejection, an increased risk of malignancy (cancer) is a recognised complication.[11] The most common cancers are non-Hodgkin’s lymphoma[citation needed] and skin cancers. The risk appears to be related to the intensity and duration of treatment.
Topical use[edit]
The most common adverse events associated with the use of topical tacrolimus ointments, especially if used over a wide area, include a burning or itching sensation on the initial applications, with increased sensitivity to sunlight and heat on the affected areas. Less common are flu-like symptoms, headache, cough, and burning eyes.[16]
Cancer risks[edit]
Tacrolimus and a related drug for eczema (pimecrolimus) were suspected of carrying a cancer risk, though the matter is still a subject of controversy. The FDA issued a health warning in March 2005 for the drug, based on animal models and a small number of patients. Until further human studies yield more conclusive results, the FDA recommends that users be advised of the potential risks. However, current practice by UK dermatologists is not to consider this a significant real concern and they are increasingly recommending the use of these new drugs.[17]
Interactions[edit]
Also like cyclosporin, it has a wide range of interactions. Tacrolimus is primarily metabolised by the cytochrome P450 system of liver enzymes, and there are many substances that interact with this system and induce or inhibit the system’s metabolic activity.[11]
Interactions include that with grapefruit which increases tacrolimus plasma concentrations. As infections are a major cause of morbidity and mortality in the post-transplant patient, the most commonly[citation needed] reported interactions include interactions with anti-microbial drugs. Macrolide antibiotics including erythromycin and clarithromycin, as well as several of the newer classes of antifungals, especially of the azole class (fluconazole, voriconazole), increase tacrolimus levels by competing for cytochrome enzymes.[11]
Pharmacology[edit]
Mechanism of action[edit]
FKBP12, the target protein of tacrolimus
In detail, tacrolimus reduces peptidylprolyl isomerase activity by binding to the immunophilin FKBP12 (FK506 binding protein), creating a new complex. This FKBP12–FK506 complex interacts with and inhibits calcineurin, thus inhibiting both T-lymphocyte signal transduction and IL-2 transcription.[19] Although this activity is similar to that of cyclosporin, the incidence of acute rejection is reduced by tacrolimus use over cyclosporin use.[3] Although short-term immunosuppression concerning patient and graft survival is found to be similar between the two drugs, tacrolimus results in a more favorable lipid profile, and this may have important long-term implications given the prognostic influence of rejection on graft survival.[20]
Pharmacokinetics[edit]
The substance is metabolized in the liver, mainly via CYP3A, and in the intestinal wall. All metabolites found in the circulation are inactive. Biological half-life varies widely and seems to be higher for healthy persons (43 hours on average) than for patients with liver transplants (12 hours) or kidney transplants (16 hours), due to differences in clearance. Tacrolimus is predominantly eliminated via the faeces in form of its metabolites.[11][21]
When applied locally on eczema, tacrolimus has little to no bioavailability.[11]
Pharmacogenetics[edit]
The predominant enzyme responsible for metabolism of tacrolimus is CYP3A5. Genetic variations within CYP3A5 that result in changes to the activity of the CYP3A5 protein can affect concentrations of tacrolimus within the body. In particular, individuals who are homozygous for the G allele at the single nucleotide polymorphism (SNP) rs776746 (also known as CYP3A5 *3/*3) have a non-functional CYP3A5 protein. The frequency of the G allele varies worldwide, from 4% in some African populations to 80–90% in Caucasian populations.[22] Across a large number of studies, individuals homozygous for the G allele have been shown to have higher concentrations of tacrolimus and require lower doses of the drug, as compared to individuals who are not homozygous for the G allele. Achieving target concentrations of tacrolimus is important – if levels are too low, then there is a risk of transplant rejection, if levels are too high, there is a risk of drug toxicities. There is evidence to suggest that dosing patients based on rs776746 genotype can result in faster and more frequent achievement of target tacrolimus levels. However, there is a lack of consistent evidence as to whether dosing based on rs776746 genotype results in improved clinical outcomes (such as a decreased risk for transplant rejection or drug toxicities), likely because patients taking tacrolimus are subject to therapeutic drug monitoring.[23][24][25][26]
Studies have shown that genetic polymorphisms of genes other than CYP3A5, such as NR1I2[27][28] (encoding PXR), also significantly influence the pharmacokinetics of tacrolimus.
History[edit]
Tacrolimus was discovered in 1987;[29] it was among the first macrolide immunosuppressants discovered, preceded by the discovery of rapamycin (sirolimus) on Rapa Nui (Easter Island) in 1975.[30] It is produced by a soil bacterium, Streptomyces tsukubaensis.[31] The name tacrolimus is derived from “Tsukuba macrolide immunosuppressant”.[32]
Available forms[edit]
The branded version of the drug is owned by Astellas Pharma, and is sold under the trade name Prograf, given twice daily. A number of other manufacturers hold marketing authorisation for alternative brands of the twice-daily formulation.[33]
Once-daily formulations with marketing authorisation include Advagraf (Astellas Pharma) and Envarsus (marketed as Envarsus XR in US by Veloxis Pharmaceuticals and marketed in Europe by Chiesi).[33] These formulations are intended to reduce pharmacokinetic variation in blood levels and facilitate compliance with dosing.
The topical formulation is marketed by LEO Pharma under the name Protopic.[33]
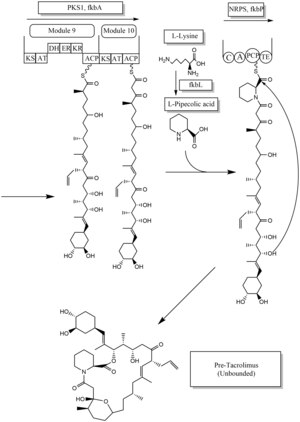
Biosynthesis[edit]
The biosynthesis of tacrolimus is hybrid synthesis of both type 1 polyketide synthases (PKS 1) and nonribosomal peptide synthases (NRPS). The research shows the hybrid synthesis consists of ten modules of type 1 polyketide synthase and one module of nonribosomal peptide synthase. The synthetic enzymes for tacrolimus are found in 19 gene clusters named fkb. The 19 genes are fkbQ, fkbN, fkbM, fkbD, fkbA, fkbP, fkbO, fkbB, fkbC, fkbL, fkbK, fkbJ, fkbI, fkbH, fkbG, allD, allR, allK and allA.[34]
There are several possible ways of biosynthesis of tacrolimus. The fundamental units for biosynthesis are following: one molecule of 4,5-dihydroxycyclohex-1-enecarboxylic acid (DHCHC) as a starter unit, four molecules of malonyl-CoA, five molecules of methylmalonyl-CoA, one molecule of allylmalonyl-CoA as elongation units. However, two molecules of malonyl-CoA are able to be replaced by two molecules of methoxymalonyl CoA. Once two malonyl-CoA molecules are replaced, post-synthase tailoring steps are no longer required where two methoxymalonyl CoA molecules are substituted. The biosynthesis of methoxymalonyl CoA to Acyl Carrier protein is proceeded by five enzymes (fkbG, fkbH, fkbI, fkbJ, and fkbK). Allylmalonyl-CoA is also able to be replaced by propionylmalonyl-CoA.[34]
The starter unit, DHCHC from the chorismic acid is formed by fkbO enzyme and loaded onto CoA-ligase domain (CoL). Then, it proceeds to NADPH dependent reduction(ER). Three enzymes, fkbA,B,C enforce processes from the loading module to the module 10, the last step of PKS 1. fkbB enzyme is responsible of allylmalonyl-CoA synthesis or possibly propionylmalonyl-CoA at C21, which it is an unusual step of general PKS 1. As mentioned, if two methoxymalonyl CoA molecules are substituted for two malonyl-CoA molecules, they will take place in module 7 and 8 (C13 and C15), and fkbA enzyme will enforce this process. After the last step (module 10) of PKS 1, one molecule of L–pipecolic acid formed from L–lysine and catalyzed through fkbL enzyme synthesizes with the molecule from the module 10. The process of L-pipecolic acid synthesis is NRPS enforced by fkbP enzyme. After synthesizing the entire subunits, the molecule is cyclized. After the cyclization, the pre-tacrolimus molecule goes through the post-synthase tailoring steps such as oxidation and S-adenosyl methionine. Particularly fkbM enzyme is responsible of alcohol methylation targeting the alcohol of DHCHC starter unit (Carbon number 31 depicted in brown), and fkbD enzyme is responsible of C9 (depicted in green). After these tailoring steps, the tacrolimus molecule becomes biologically active.[34][35][36]
See also[edit]
References[edit]
- ^ Berdoulay A, English RV, Nadelstein B (2005). “Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dogs with keratoconjunctivitis sicca”. Veterinary Ophthalmology. 8 (4): 225–32. doi:10.1111/j.1463-5224.2005.00390.x. PMID 16008701.
- ^ “Tacrolimus for Dogs and Cats”.
- ^ Jump up to:a b McCauley, Jerry (2004-05-19). “Long-Term Graft Survival In Kidney Transplant Recipients”. Slide Set Series on Analyses of Immunosuppressive Therapies. Medscape. Retrieved 2006-06-06.
- ^ Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL (October 2006). McAlister V (ed.). “Cyclosporin versus tacrolimus for liver transplanted patients”. The Cochrane Database of Systematic Reviews. 4 (4): CD005161. doi:10.1002/14651858.CD005161.pub2. PMID 17054241.
- ^ O’Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A (October 2002). “Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial”. Lancet. 360 (9340): 1119–25. doi:10.1016/S0140-6736(02)11196-2. PMID 12387959.
- ^ Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU (May 2006). “Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease–a long-term follow-up”. The American Journal of Gastroenterology. 101 (5): 1048–56. doi:10.1111/j.1572-0241.2006.00524.x. PMID 16573777.
- ^ Baumgart DC, Macdonald JK, Feagan B (July 2008). Baumgart DC (ed.). “Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis”. The Cochrane Database of Systematic Reviews. 16 (3): CD007216. doi:10.1002/14651858.CD007216. PMID 18646177.
- ^ Jump up to:a b Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Protopic.
- ^ Silverberg NB, Lin P, Travis L, Farley-Li J, Mancini AJ, Wagner AM, Chamlin SL, Paller AS (November 2004). “Tacrolimus ointment promotes repigmentation of vitiligo in children: a review of 57 cases”. Journal of the American Academy of Dermatology. 51 (5): 760–6. doi:10.1016/j.jaad.2004.05.036. PMID 15523355.
- ^ Singh JA, Hossain A, Kotb A, Wells G (September 2016). “Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis”. BMC Medicine. 14 (1): 137. doi:10.1186/s12916-016-0673-8. PMC 5022202. PMID 27623861.
- ^ Jump up to:a b c d e f g h Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Prograf.
- ^ Fukatsu S, Fukudo M, Masuda S, Yano I, Katsura T, Ogura Y, Oike F, Takada Y, Inui K (April 2006). “Delayed effect of grapefruit juice on pharmacokinetics and pharmacodynamics of tacrolimus in a living-donor liver transplant recipient”. Drug Metabolism and Pharmacokinetics. 21 (2): 122–5. doi:10.2133/dmpk.21.122. PMID 16702731.
- ^ Naesens M, Kuypers DR, Sarwal M (February 2009). “Calcineurin inhibitor nephrotoxicity” (PDF). Clinical Journal of the American Society of Nephrology. 4 (2): 481–508. doi:10.2215/CJN.04800908. PMID 19218475.
- ^ Miwa Y, Isozaki T, Wakabayashi K, Odai T, Matsunawa M, Yajima N, Negishi M, Ide H, Kasama T, Adachi M, Hisayuki T, Takemura T (2008). “Tacrolimus-induced lung injury in a rheumatoid arthritis patient with interstitial pneumonitis”. Modern Rheumatology. 18 (2): 208–11. doi:10.1007/s10165-008-0034-3. PMID 18306979.
- ^ O’Donnell MM, Williams JP, Weinrieb R, Denysenko L (2007). “Catatonic mutism after liver transplant rapidly reversed with lorazepam”. General Hospital Psychiatry. 29 (3): 280–1. doi:10.1016/j.genhosppsych.2007.01.004. PMID 17484951.
- ^ Hanifin JM, Paller AS, Eichenfield L, Clark RA, Korman N, Weinstein G, Caro I, Jaracz E, Rico MJ (August 2005). “Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis”. Journal of the American Academy of Dermatology. 53 (2 Suppl 2): S186–94. doi:10.1016/j.jaad.2005.04.062. PMID 16021174.
- ^ N H Cox & Catherine H Smith (December 2002). “Advice to dermatologists re topical tacrolimus” (PDF). Therapy Guidelines Committee. British Association of Dermatologists. Archived from the original (PDF) on 2013-12-13.
- ^ William F. Ganong (2005-03-08). Review of medical physiology(22nd ed.). Lange medical books. p. 530. ISBN 978-0-07-144040-0.
- ^ Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL (August 1991). “Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes”. Cell. 66 (4): 807–15. doi:10.1016/0092-8674(91)90124-H. PMID 1715244.
- ^ Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, Darwish M, Mulhem A, Ojjeh A, Almawi WY (September 2005). “Tacrolimus (FK506) versus cyclosporine microemulsion (neoral) as maintenance immunosuppression therapy in kidney transplant recipients”. Transplantation Proceedings. 37 (7): 3025–8. doi:10.1016/j.transproceed.2005.08.040. PMID 16213293.
- ^ Jump up to:a b Dinnendahl, V; Fricke, U, eds. (2003). Arzneistoff-Profile (in German). 9 (18 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ Bains, Ripudaman Kaur. “Molecular diversity and population structure at the CYP3A5 gene in Africa” (PDF). University College London. Retrieved 13 June 2016.
- ^ Staatz CE, Tett SE (2004). “Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation”. Clinical Pharmacokinetics. 43 (10): 623–53. doi:10.2165/00003088-200443100-00001. PMID 15244495.
- ^ Staatz CE, Goodman LK, Tett SE (March 2010). “Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I”. Clinical Pharmacokinetics. 49 (3): 141–75. doi:10.2165/11317350-000000000-00000. PMID 20170205.
- ^ Staatz CE, Goodman LK, Tett SE (April 2010). “Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II”. Clinical Pharmacokinetics. 49 (4): 207–21. doi:10.2165/11317550-000000000-00000. PMID 20214406.
- ^ Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB (October 2013). “PharmGKB summary: cyclosporine and tacrolimus pathways”. Pharmacogenetics and Genomics. 23(10): 563–85. doi:10.1097/fpc.0b013e328364db84. PMC 4119065. PMID 23922006.
- ^ Benkali K, Prémaud A, Picard N, Rérolle JP, Toupance O, Hoizey G, Turcant A, Villemain F, Le Meur Y, Marquet P, Rousseau A (2009-01-01). “Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients”. Clinical Pharmacokinetics. 48 (12): 805–16. doi:10.2165/11318080-000000000-00000. PMID 19902988.
- ^ Choi Y, Jiang F, An H, Park HJ, Choi JH, Lee H (January 2017). “A pharmacogenomic study on the pharmacokinetics of tacrolimus in healthy subjects using the DMETTM Plus platform”. The Pharmacogenomics Journal. 17 (1): 105–106. doi:10.1038/tpj.2016.85. PMID 27958377.
- ^ Hatanaka H, Iwami M, Kino T, Goto T, Okuhara M (November 1988). “FR-900520 and FR-900523, novel immunosuppressants isolated from a Streptomyces. I. Taxonomy of the producing strain”. The Journal of Antibiotics. 41 (11): 1586–91. doi:10.7164/antibiotics.41.1586. PMID 3198493.
- ^ Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H (September 1987). “FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics”. The Journal of Antibiotics. 40 (9): 1249–55. doi:10.7164/antibiotics.40.1249. PMID 2445721.
- ^ Pritchard DI (May 2005). “Sourcing a chemical succession for cyclosporin from parasites and human pathogens”. Drug Discovery Today. 10 (10): 688–91. doi:10.1016/S1359-6446(05)03395-7. PMID 15896681. Supports source organism, but not team information
- ^ Ponner, B, Cvach, B (Fujisawa Pharmaceutical Co.): Protopic Update 2005
- ^ Jump up to:a b c Joint Formulary Committee. “British National Formulary (online)”. London: BMJ Group and Pharmaceutical Press. Retrieved 24 September 2015.
- ^ Jump up to:a b c Ordóñez-Robles M, Santos-Beneit F, Martín JF (May 2018). “omic Approaches”. Antibiotics. 7 (2): 39. doi:10.3390/antibiotics7020039. PMC 6022917. PMID 29724001.
- ^ Chen D, Zhang L, Pang B, Chen J, Xu Z, Abe I, Liu W (May 2013). “FK506 maturation involves a cytochrome p450 protein-catalyzed four-electron C-9 oxidation in parallel with a C-31 O-methylation”. Journal of Bacteriology. 195 (9): 1931–9. doi:10.1128/JB.00033-13. PMC 3624582. PMID 23435975.
- ^ Mo S, Ban YH, Park JW, Yoo YJ, Yoon YJ (December 2009). “Enhanced FK506 production in Streptomyces clavuligerus CKD1119 by engineering the supply of methylmalonyl-CoA precursor”. Journal of Industrial Microbiology & Biotechnology. 36(12): 1473–82. doi:10.1007/s10295-009-0635-7. PMID 19756799.
External links
These papers does not mention eye issues:
Br J Dermatol. 2008 Sep;159(4):942-51. doi: 10.1111/j.1365-2133.2008.08747.x. Epub 2008 Jul 15.
A 4-year follow-up study of atopic dermatitis therapy with 0.1% tacrolimus ointment in children and adult patients.
Reitamo S1, Rustin M, Harper J, Kalimo K, Rubins A, Cambazard F, Brenninkmeijer EE, Smith C, Berth-Jones J, Ruzicka T, Sharpe G, Taieb A; 0.1% Tacrolimus Ointment Long-term Follow-up Study Group.
Abstract
BACKGROUND:
For the treatment of a chronic disease like atopic dermatitis, sustained tolerability and efficacy of the applied medication are essential.
OBJECTIVES:
The present open-label, noncomparative study was conducted to obtain information on the long-term safety and efficacy of 0.1% tacrolimus ointment.
METHODS:
Patients aged 2 years or older with an affected body surface area of more than 5%, who previously participated in a clinical trial on tacrolimus ointment, were eligible for this study. The treatment area was defined by the investigator at study entry. Both children and adults applied continuously or intermittently 0.1% tacrolimus ointment twice daily during episodes of active disease plus an additional week after remission over a follow-up period of up to 4 years.
RESULTS:
The intent-to-treat population comprised 782 patients, with a median age of 22 years (range 2-72). Patients remained in the study for up to 4 years. Approximately half of the patients discontinued the study prematurely; the median follow-up was 1422 days. Median tacrolimus ointment use was 31.2 g during the first week; ointment use decreased during the first year and then remained stable for the remainder of the study. The median cumulative tacrolimus use was 271.5 g at month 6, 462.5 g at month 12, 739.9 g at month 24, 1029.3 g at month 36 and 1320.8 g at month 48. Altogether 51.8% of patients discontinued the study prematurely; the main reasons were withdrawal of consent (13.3%), loss to follow-up (11.3%) and lack of efficacy (9.4%). Adverse events led to study discontinuation in 3.7% of the patients. The most frequent application site events were skin burning and pruritus. These events were most often reported in adult patients during the initial treatment period; prevalence decreased after the first week and remained at a low level throughout the study. Nonapplication site events occurred with stable incidences throughout the study period. In general, calculated daily hazard rates did not indicate an increased risk of adverse events with prolonged treatment. The total affected body surface area decreased substantially upon onset of treatment and efficacy of treatment was maintained until the end of the study with smaller but continuous improvements throughout the follow-up period. Overall, 75% of the patients and 76% of the investigators rated their satisfaction with the treatment as excellent, very good or good at the end of the study or at the time of premature discontinuation.
CONCLUSIONS:
The safety profile of intermittent or continuous long-term application of 0.1% tacrolimus ointment for up to 4 years was consistent with that which has been established from shorter studies and gave no reason for concern. In addition, 0.1% tacrolimus ointment demonstrated sustained efficacy as reflected by the expression of high satisfaction with treatment by both patients and investigators.
Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis
Objective
This study was designed to evaluate the long-term safety and efficacy of 0.1% tacrolimus ointment in adult and pediatric patients with atopic dermatitis (AD).
Methods
A total of 408 adult and 391 pediatric patients with AD who had participated in a previous clinical trial of tacrolimus ointment were enrolled in this long-term, open-label, noncomparative trial. Tacrolimus ointment 0.1% was applied twice daily either intermittently or continuously to the affected areas. Efficacy and safety assessments included percent body surface area affected, Eczema Area and Severity Index score, individual signs of AD, and the incidence of adverse events.
Results
A total of 799 patients were evaluated, of whom 300 (37.5%) were followed for more than 3 years (maximum 49 months). Improvements in efficacy parameters were observed within 1 week of treatment and continued for the duration of the study. Common adverse events included skin burning, pruritus, skin infection, skin erythema, flu-like symptoms, and headache. The incidence of adverse events, including cutaneous infections, did not increase with time on study.
Conclusion
Tacrolimus ointment therapy is a rapidly effective and safe treatment for the management of AD in pediatric and adult patients for up to 4 years.
ELECTRONIC ARTICLES
Tacrolimus Ointment 0.03% Is Safe and Effective for the Treatment of Mild to Moderate Atopic Dermatitis in Pediatric Patients: Results From a Randomized, Double-Blind, Vehicle-Controlled Study
Lawrence A. Schachner, Cindy Lamerson, Mary P. Sheehan, Mark Boguniewicz, Joy Mosser, Sharon Raimer, Toni Shull, Eileen Jaracz and ; for the US Tacrolimus Ointment Study Group
Pediatrics September 2005, 116 (3) e334-e342; DOI: https://doi.org/10.1542/peds.2004-2638
Abstract
Objective. This study was designed to compare the safety and efficacy of tacrolimus ointment 0.03% with vehicle ointment for the treatment of mild to moderate atopic dermatitis (AD) in pediatric patients.
Methods. A total of 317 patients (2–15 years of age) with mild to moderate AD were randomized to receive tacrolimus ointment or vehicle ointment twice daily in a 6-week, multicenter, double-blind study. Efficacy evaluations, including the Investigators’ Global Atopic Dermatitis Assessment, eczema area and severity index, percentage of total body surface area affected, and patient assessment of itch occurred at baseline, day 4, and weeks 2, 4, and 6. Cutaneous adverse events were recorded to evaluate safety.
Results. At the end of study, 50.6% (80 of 158) of the patients were treated successfully with tacrolimus ointment based on Investigators’ Global Atopic Dermatitis Assessment scores, a significant improvement compared with patients treated with vehicle ointment (25.8% [41 of 159]). The percent improvement from baseline in eczema area and severity index scores was also significantly greater in tacrolimus-treated patients (54.8%) compared with vehicle-treated patients (20.8%). There was also a significant improvement in the percentage of total body surface area affected of tacrolimus-treated patients (50.5% reduction from baseline) compared with vehicle-treated patients (16.4%). Patient itch scores were significantly lower in tacrolimus-treated patients (2.1) versus vehicle-treated patients (3.7). Overall, the incidence of cutaneous adverse events reported was similar for both treatment groups. There was no significant difference in the incidence of burning or stinging between treatment groups. Significantly fewer tacrolimus-treated patients prematurely discontinued from the study because of a cutaneous adverse event in the treatment area or experienced increased itching and erythema at the application site.
Conclusion. Monotherapy with tacrolimus ointment 0.03% is a safe and effective treatment alternative for pediatric patients with mild to moderate AD.