What is the Risk of Infection After Autologous Serum? Close to 0%
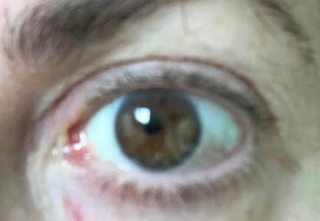
Mild yellow discharge noted in the nasal cornea of left eye
Autologous Serum (AS) eye drops have been recommended for treatment of patients with several ocular surface disturbances, such as
1. Sjögren’s syndrome-related tear deficiency,
2. non-Sjögren’s tear deficiency associated with graft-versus-host disease,
3. Neurotrophic keratitis,
4. Persistent epithelial defects,
5. Superior limbic keratoconjunctivitis,
6. Postoperative dry eye induced by LASIK.
7. Severe dry eye from Meibomian gland dysfunction or other autoimmune disease.
People treated with 20% to 50% AS four to eight times a day have reported subjective improvement in dry eye symptoms; investigators have also noted objective improvement based on fluorescein staining and results of break-up time tests (Chiang 2007; Hyon 2007; Kojima 2005b; Matsumoto 2004; Ogawa 2003; Poon 2001; Tananuvat 2001).
Studies have shown that AS contain multiple growth factors and biochemical factors, such as EGF, vitamin A, TGF-β, fibronectin, substance P, insulin-like growth factor-1 (IGF-1), nerve growth factor (NGF), and other cytokines essential for proliferation, differentiation, and maturation of the normal ocular surface epithelium (Gordon 1995; Matsumoto 2004; McCluskey 1987; Nishida 1983; Nishida 1987; Phan 1987; Poon 2001). The even have been reported to contain a patient’s own stem cells. Therefore, a potential advantage of AS over traditional therapies is that AS serves as a lacrimal substitute that provides lubrication and other biochemical components of tears to assist in corneal and conjunctival epithelium maintenance with limited toxicity (Dogru 2011; Geerling 2004; Liu 2005; Poon 2001; Quinto 2008).
I have used Autologous Serum since about 2000 starting at Harvard Medical School and now in private practice at Visionary Eye Doctors. While I have heard of the possibility of having a corneal ulcer from Autologous Serum, I have never seen one, nor could I find a report of a published case on Pubmed. In the reports below, there is only 1 case of inflammation at the limbus (the location where the cornea becomes the white part of the eye/sclera), called limbitis and 2 cases of conjunctivitis from AS. There are no published report of corneal ulcers. The other studies tested contamination in bottles of AS but found no infection in any patients.
Complications
AS usually are well tolerated, and most recipients report less discomfort. Occasionally, patients may experience increased discomfort, slight epitheliopathy (dropout of corneal epithelial cells, akin to fluorescein staining of the surface of the eye), bacterial conjunctivitis, or eyelid eczema (
Ogawa 2003;
Rocha 2000;
Tananuvat 2001).
Fox 1984 reported no serious complications but mentioned that other investigators had encountered scleral vasculitis and melting in people with rheumatoid arthritis.
McDonnell 1988 described complications such as the deposit of immunoglobulins within the cornea and the presence of corneal peripheral infiltrates with 100% autologous serum treatment in one person.
Risk of infection
Some components of serum may have bacteriostatic effects, for example, lysozyme, complement, and IgG; therefore, the addition of another bacteriostatic agent may not be necessary. It has been reported that AS can be used safely in both outpatient and inpatient settings, under a strict protocol of preparation and storage (
Langnado 2004;
Partal 2011). However, even though AS are prepared under sterile conditions on an individual patient basis, researchers have noted risks for contamination and consequent infection during preparation, storage, and use of the drops (
Geerling 2004;
Lee 2008).
Recently, a lovely patient with a history of chronic Lyme Disease recently came in on a Saturday with complaints of yellow discharge in her left eye after using Autologous Serum. She has a history of migraines and takes Imitrex for this at times. She said that a couple of hours of using her Autologous Serum, (her own serum), she got a terrible headache and had to lay down. She was out of work for a day or two because of the headache. Soon after she had discharge in her left eye as shown above. The doctor on call did not culture the discharge, but she was given Tobradex and the discharge went away. She did not have any corneal ulcer.
I suspected that her symptoms were not from the AS as her symptoms were really only in the left eye, and she was using AS in both eyes. Still she does have a history of Lyme disease and has a history of significant sensitivity to drops with similar reactions in the past. For now, we will avoid using AS in this patient.
If you have a patient with a history of Lyme who also had such a negative reaction to their own blood, please let me know.
Sandra Lora Cremers, MD, FACS
Autologous serum eye drops for dry eye.
Abstract
BACKGROUND:
Theoretically, autologous serum eye drops (AS) offer a potential advantage over traditional therapies on the assumption that AS not only serve as a lacrimal substitute to provide lubrication but contain other biochemical components that allow them to mimic natural tears more closely. Application of AS has gained popularity as second-line therapy for patients with dry eye. Published studies on this subject indicate that autologous serum could be an effective treatment for dry eye.
OBJECTIVES:
We conducted this review to evaluate the efficacy and safety of AS given alone or in combination with artificial tears as compared with artificial tears alone, saline, placebo, or no treatment for adults with dry eye.
SEARCH METHODS:
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 5), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2016), Embase (January 1980 to July 2016), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to July 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We also searched the Science Citation Index Expanded database (December 2016) and reference lists of included studies. We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 5 July 2016.
SELECTION CRITERIA:
We included randomized controlled trials (RCTs) that compared AS versus artificial tears for treatment of adults with dry eye.
DATA COLLECTION AND ANALYSIS:
Two review authors independently screened all titles and abstracts and assessed full-text reports of potentially eligible trials. Two review authors extracted data and assessed risk of bias and characteristics of included trials. We contacted investigators to ask for missing data. For both primary and secondary outcomes, we reported mean differences with corresponding 95% confidence intervals (CIs) for continuous outcomes. We did not perform meta-analysis owing to differences in outcome assessments across trials.
MAIN RESULTS:
We identified five eligible RCTs (92 participants) that compared AS versus artificial tears or saline in individuals with dry eye of various origins (Sjögren’s syndrome-related dry eye, non-Sjögren’s syndrome dry eye, and postoperative dry eye induced by laser-assisted in situ keratomileusis (LASIK)). We assessed the certainty of evidence as low or very low because of lack of reporting of quantitative data for most outcomes and unclear or high risk of bias among trials. We judged most risk of bias domains to have unclear risk in two trials owing to insufficient reporting of trial characteristics, and we considered one trial to have high risk of bias for most domains. We judged the remaining two trials to have low risk of bias; however, these trials used a cross-over design and did not report data in a way that could be used to compare outcomes between treatment groups appropriately. Incomplete outcome reporting and heterogeneity among outcomes and follow-up periods prevented inclusion of these trials in a summary meta-analysis.Three trials compared AS with artificial tears; however, only one trial reported quantitative data for analysis. Low-certainty evidence from one trial suggested that AS might provide some improvement in participant-reported symptoms compared with artificial tears after two weeks of treatment; the mean difference in mean change in symptom score measured on a visual analogue scale (range 0 to 100, with higher scores representing worse symptoms) was -12.0 (95% confidence interval (CI) -20.16 to -3.84; 20 participants). This same trial found mixed results with respect to ocular surface outcomes; the mean difference in mean change in scores between AS and artificial tears was -0.9 (95% CI -1.47 to -0.33; 20 participants; low-certainty evidence) for fluorescein staining and -2.2 (95% CI -2.73 to -1.67; 20 participants; low-certainty evidence) for Rose Bengal staining. Both staining scales range from 0 to 9, with higher scores indicating worse results. The mean change in tear film break-up time was 2.00 seconds longer (95% CI 0.99 to 3.01; 20 participants; low-certainty evidence) in the AS group than in the artificial tears group. Investigators reported no clinically meaningful differences in Schirmer’s test scores between groups (mean difference -0.40 mm, 95% CI -2.91 to 2.11; 20 participants; low-certainty evidence). None of these three trials reported tear hyperosmolarity and adverse events.Two trials compared AS versus saline; however, only one trial reported quantitative data for analysis of only one outcome (Rose Bengal staining). Trial investigators of the two studies reported no differences in symptom scores, fluorescein staining scores, tear film break-up times, or Schirmer’s test scores between groups at two to four weeks’ follow-up. Very low-certainty evidence from one trial suggested that AS might provide some improvement in Rose Bengal staining scores compared with saline after four weeks of treatment; the mean difference in Rose Bengal staining score (range from 0 to 9, with higher scores showing worse results) was -0.60 (95% CI -1.11 to -0.09; 35 participants). Neither trial reported tear hyperosmolarity outcomes. One trial reported adverse events; two of 12 participants had signs of conjunctivitis with negative culture that did resolve.
AUTHORS’ CONCLUSIONS:
Overall, investigators reported inconsistency in possible benefits of AS for improving participant-reported symptoms and other objective clinical measures. There might be some benefit in symptoms with AS compared with artificial tears in the short-term, but we found no evidence of an effect after two weeks of treatment. Well-planned, large, high-quality RCTs are warranted to examine participants with dry eye of different severities by using standardized questionnaires to measure participant-reported outcomes, as well as objective clinical tests and objective biomarkers to assess the benefit of AS therapy for dry eye.
Case Reports in Ophthalmological Medicine
Volume 2011 (2011), Article ID 576521, 3 pages
http://dx.doi.org/10.1155/2011/576521
Case Report
Limbitis Secondary to Autologous Serum Eye Drops in a Patient with Atopic Keratoconjunctivitis
Illinois Eye and Ear Infirmary, University of Illinois at Chicago, 1855 West Taylor Street, Chicago, IL 60612, USA
Received 3 November 2011; Accepted 1 December 2011
Academic Editors: S.-J. Chen, S. Machida, and N. Rosa
Copyright © 2011 Jeffrey David Welder et al. This is an open access article distributed under the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose. Report a case of limbitis secondary to autologous serum eye drops in a patient with atopic keratoconjunctivitis. Design. Interventional case report. Methods. A 32-year-old African American female with atopic keratoconjunctivitis (AKC) presented with chronic dry eye and diffuse punctate epithelial erosions refractory to conservative treatment. She was initially managed with cyclosporine ophthalmic 0.05% in addition to preservative-free artificial tears and olopatadine hydrochloride 0.2% for 6 months. She was later placed on autologous serum eye drops (ASEDs) and 4 weeks into treatment developed unilateral limbitis. The limbitis resolved shortly after stopping ASEDs in that eye; however, the drops were continued in the contralateral eye, which subsequently developed limbitis within 2 weeks. ASEDs were discontinued in both eyes, and the patient has remained quiet ever since. Results. Patient with a history of AKC and no prior history of limbitis developed limbitis shortly after starting ASEDs, which resolved promptly after discontinuation of therapy with no subsequent recurrence of inflammation. Conclusion. ASEDs are widely used in the treatment of complicated or treatment refractory dry eye. The potential side effects should be kept in mind when prescribing ASEDs for any patient, especially in those with underlying immunological diseases and circulating inflammatory factors.
Cornea. 2013 Aug;32(8):1116-9. doi: 10.1097/ICO.0b013e3182910036.
Contamination risk of 100% autologous serum eye drops in management of ocular surface diseases.
Abstract
PURPOSE:
To evaluate the sterility and safety of 100% nonpreserved, autologous, serum eye drop treatment in patients with ocular surface diseases.
METHODS:
A total of 147 autologous serum bottles (294 samples) from 21 patients with ocular surface diseases were included. Seven bottles with autologous serum were prepared for each patient, and each bottle was used for only 1 day, then discarded. Two samples from each bottle were taken (before use and after 24 hours of use) and then cultured in standard media. The culture plates were held for at least 6 weeks or until no growth could be confirmed. To monitor safety, all patients were admitted and evaluated for the occurrence of infection.
RESULTS:
In the pretreatment group, 4 samples from 4 patients (1.36%) were positive for bacteria and 7 samples from 7 patients (2.38%) were positive for fungi. In the 24-hour-after-treatment group, 1 culture (0.34%) was positive for bacteria, and 6 samples (2.04%) from 6 patients were positive for fungi. Aspergillus spp and Fonsecaea spp were the most common organisms identified in any of the cultures. Neither clinical nor microbiological evidence of infection was demonstrated in any patient during the treatment or follow-up periods.
CONCLUSIONS:
Although no ocular surface infection was observed, the patients under 100% autologous serum eye drops therapy should be closely monitored for clinical evidence of fungal and bacterial infections.
A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders.
Abstract
AIM:
To assess microbial contamination of 20% autologous serum (AS) eye drops used in a hospital inpatient setting.
METHOD:
14 patients received autologous serum drops from 4 to 14 days with a cumulative total of 67 days. For each day the first and last drop (total 134 samples) was cultured on broth and blood agar.
RESULTS:
Four patients (9 samples) grew Staphylococcus epidermidis only. One patient (1 sample) showed Staphylococcus epidermidis and a scanty growth of viridans streptococci in the same sample, and on different days the same patient grew Staphylococcus aureus in one sample and Staphylococcus epidermidis in another sample. One patient (1 sample) grew micrococcus. There was no clinical or microbial evidence of infection in any of these six patients.
CONCLUSION:
This study shows that autologous serum drops can be safely used in an inpatient setting, under a strict protocol of preparation and storage, without significant risk of bacterial contamination and consequent infection.